Figure 1.
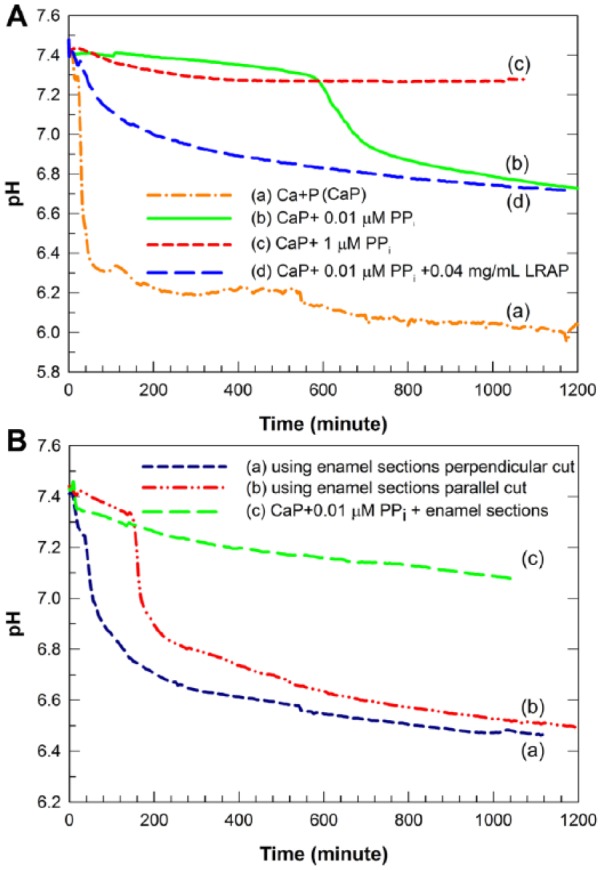
Changes in pH of supersaturated calcium phosphate (CaP) solutions containing 1.3 mM Ca, 1.5 mM P, 50 mM NaCl, pHi = 7.4 as a function of time under various conditions. (A) In the absence of acid-etched enamel: Control, without added inorganic pyrophosphate (PPi) and leucine-rich amelogenin peptide (LRAP), a marked decrease in solution pH was observed (a). The mineralization solution was stabilized by the addition of 0.01 µM PPi for up to 10 h (b) while the same solution was stabilized by the addition of 1 µM PPi for up to 20 h (c). In the presence of 0.04 mg/mL LRAP, the 0.01-µM PPi-stabilized mineral solution exhibited a slight drop in pH within the first 2 h that was followed by a more gradual decrease to pH 6.7 over the 20-h experimental time period (d). (B) In the presence of acid-etched enamel: With 0.04 mg/mL LRAP and 3 acid-etched enamel sections cut in a direction perpendicular to enamel rods (a) and 3 acid-etched enamel sections cut parallel to enamel rods (b) in the PPi-stabilized mineralizing solution, significant decreases in pH were observed within 40 to 50 and 180 min, respectively. As a control, the 0.01-µM PPi-stabilized mineral solution in the presence of enamel sections cut in a direction perpendicular to enamel rods but without LRAP showed a gradual and slight pH decrease to pH 7.1 over 20 h (c).
