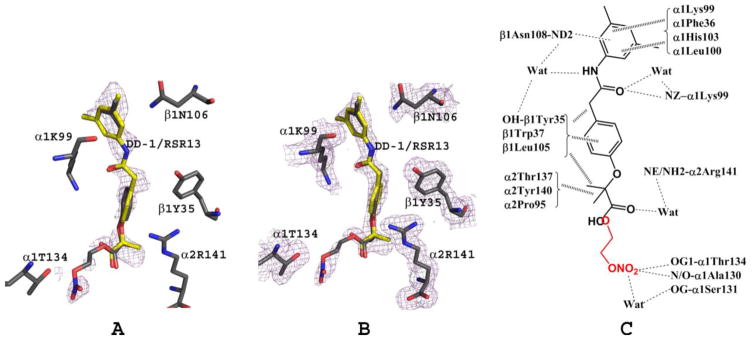
Figure 5.
DD-1/RSR13 interactions at the central water cavity (α1α2β1 binding site) of bis-ligated T-state HbNO. Note that RSR13 (yellow) and DD-1 (grey) occupy the same site of the α1α2β1 binding site and each was refined with 40% occupancy. (A) Difference electron density (Fo-Fc) map of bound DD-1 and RSR13 (before DD-1/RSR13 were built into the model), contoured at 2.5σ. (B) Final 2Fo-Fc map of bound DD-1 and RSR13, contoured at 1.0σ. The two maps are superimposed with the final refined DD-1/RSR13 model. For clarity, not all interacting residues are shown. (C) Schematic representation of interactions between DD-1/RSR13 and the protein.