Abstract
Practice effects on neuropsychological tests, which are improvements in test scores due to repeated exposure to testing materials, are robust in healthy elders, but muted in older adults with cognitive disorders. Conversely, few studies have investigated practice effects on motor tasks involving procedural memory, particularly across test-retest periods exceeding 24 hours. The current study examined one-week practice effects on a novel upper extremity motor task in 54 older adults with amnestic Mild Cognitive Impairment. Results indicate that these individuals with primary memory deficits did improve on this motor task within a brief training session as well as across one week. These practice effects were unrelated to demographic characteristics or global cognition. One-week practice effects were, however, negatively related to delayed memory function, with larger practice effects being associated with poorer delayed memory and potentially better visuospatial ability. The presence of longer-term practice effects on a procedural motor task not only has implications for how longitudinal assessments with similar measures involving implicit memory might be interpreted, but may also inform future rehabilitative strategies for patients with more severe declarative memory deficits.
Keywords: Mild Cognitive Impairment, procedural memory, motor learning, practice effects, aging
INTRODUCTION
Practice effects are improvements in test scores due to repeated exposure to the testing materials. They are widely observed during serial neuropsychological assessments, and can lead to misinterpretations of longitudinal cognitive studies. In a recent meta-analysis, Calamia et al. (2012) showed that practice effects are complex, being influenced by demographic variables (e.g., older age and lower education have smaller practice effects), study design (e.g., a longer retest interval and the use of alternate forms leads to smaller practice effects), and clinical condition (e.g., patients with Mild Cognitive Impairment and Alzheimer’s disease have smaller practice effects). This meta-analysis also demonstrated that different cognitive domains exhibit differential practice effects. For example, visual memory tests tend to have larger practice effects than tests tapping other cognitive domains.
Practice effects have been documented on numerous declarative memory tests, such as the Hopkins Verbal Learning Test for example (Duff, Callister, Dennett, & Tometich, 2012). In patients with Mild Cognitive Impairment (e.g., Darby, Maruff, Collie, & McStephen, 2002) or Alzheimer’s disease (e.g., Cooper et al., 2001), data collectively support that the worse the cognitive impairment, the smaller the practice effect is. Compared to declarative memory, however, much less is known about practice effects on tests of procedural (i.e., motor) memory. In non-demented older adults, within-session and 24-hour practice effects have been reported on serial reaction time tests (Brown, Robertson, & Press, 2009; Howard, Howard, Dennis, & Kelly, 2008) and a novel upper extremity motor task involving point-to-point reaching (Schaefer & Duff, 2015). Interestingly, within-session and 24-hour practice effects in adults with Mild Cognitive Impairment and Alzheimer’s Disease appear to be equivalent to those in non-demented age-matched adults on a variety of motor tasks (Dick, Nielson, Beth, Shankle, & Cotman, 1995; Rouleau, Salmon, & Vrbancic, 2002; Yan & Dick, 2006). Even studies of motor skill learning following extensive training in cognitively impaired populations, such as Alzheimer’s disease, show comparable rates and amounts of motor learning relative cognitively intact control groups (e.g., Dick et al., 1995; Jacobs et al., 1999). These findings within procedural memory collectively suggest, therefore, that cognitively impaired adults will show motor practice effects over a longer test-retest interval as well. The existing literature in other memory domains, however, appears to suggest conflicting hypotheses about whether older adults with Mild Cognitive Impairment will, or will not, show one-week (or longer) practice effects on a motor task, since one-week (or longer) practice effects on declarative tasks are attenuated or minimal in these individuals (Duff et al., 2008; Duff et al., 2011). Thus, the primary purpose of this study was to test whether older adults with Mild Cognitive Impairment would show one-week motor practice effects in addition to known within-session practice effects.
As noted earlier, practice effects on other neuropsychological tests appear to be moderated by a number of different variables. Some demographic variables such as age and education, and cognitive variables such as intelligence, can influence the amount of practice observed on neuropsychological tests (Calamia, Markon, & Tranel, 2012). For example, Rapport et al. (1997) reported that the ‘rich get richer,’ such that those with higher levels of intelligence showed larger practice effects on cognitive tests. Conversely, other studies have reported that practice effects are not related to other cognitive domains (Duff, Callister, Dennett, & Tometich, 2012), thereby raising the question of what might predict whether an individual will show practice effects on a given task, be they over a short or longer test-retest interval. Thus, a secondary purpose of this study was to investigate if demographic (e.g., age, education, or gender) or cognitive (e.g. immediate memory or attention) variables predicted within-session and one-week motor practice effects in older adults with Mild Cognitive Impairment. Previous studies in motor practice effects in cognitively impaired adults have only described but not predicted such effects, regardless of test-retest interval. Based existing practice effects literature in other non-motor neuropsychological testing (e.g., Calamia et al., 2012), however, we hypothesized that older participants with lower levels of education and lower global cognitive scores would show smaller practice effects.
METHODS
Participants
All human research procedures were approved by the University of Utah Institutional Review Board, in accordance with the Helsinki Declaration. Fifty-four older adults were recruited from a cognitive disorders clinic, independent living facilities, or community presentations, and provided informed consent prior to enrollment. All individuals presented with memory complaints and objective memory deficits, but no significant functional limitations (e.g., still driving, managing medications, handling finances), as confirmed by a licensed neuropsychologist). As such, they met criteria for Mild Cognitive Impairment, primarily of the amnestic subtype according to Winblad et al. (2004). Subjective memory complaints were reported by participants and/or knowledgeable collaterals. Objective memory problems were determined by a significant discrepancy between delayed recall measures and an estimate of premorbid intellect (see additional description below in “Cognitive Assessment”). Additionally, participants had to be 65 years or older and have adequate vision, hearing, and motor abilities to complete cognitive testing. Exclusion criteria included medical comorbidities likely to affect cognition (e.g., history of major neurological disorders, major psychiatric disorders, or substance abuse); use of anticonvulsant or antipsychotic medications; Exclusion criteria included <65 years old, history of major neurological (e.g., stroke, seizure, traumatic brain injury) or psychiatric (e.g., bipolar disorder, schizophrenia) disorder, history of substance abuse, use of anticonvulsant or antipsychotic medication, current severe depression as indicated by scores of >6 on the 15-item Geriatric Depression Scale (GDS) or >14 on the 30-item GDS, current residence in a skilled-nursing facility, or any evidence of clear functional impairment in daily activities.
Experimental methods and design
Cognitive assessment
As part of a more comprehensive cognitive assessment, all participants completed the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Randolph, 1998), which is a brief, individually administered test measuring attention, language, visuospatial/constructional abilities, and immediate and delayed memory. It consists of 12 subtests, which yield five Index scores and a Total Scale score. Normative information from the manual is used to calculate the Index and Total scores, which are age-corrected standard scores (Mean=100, SD = 15). All subtests were administered and scored as defined in the manual, with the exception of the Figure Copy and Figure Recall, which is more fully described elsewhere (Duff et al., 2007). An estimate of premorbid intellect (Reading subtest of the Wide Range Achievement Test – 4, WRAT-4) and a screening of depressive symptoms (30-item Geriatric Depression Scale, GDS) were also administered. The WRAT-4 Reading subtest scores reported in this study were age-corrected standard scores. Participants were considered as having amnestic Mild Cognitive Impairment based on their performance on three memory tests: RBANS Delayed Memory Index (DMI), Hopkins Verbal Learning Test – Revised (HVLT-R) Delay Recall, and Brief Visuospatial Memory Test – Revised (BVMT-R) Delay Recall. Of these three tests, the HVLT-R and BVMT-R are more sensitive (Duff, Hobson, Beglinger, & O’Bryant, 2010) in detecting patterns of cognitive impairment. To be considered as having Mild Cognitive Impairment of the amnestic subtype, all participants were required to have 1) at least one very poor score on at least one memory measure compared to his/her premorbid IQ (defined as >−2 SD) despite having average scores on the remaining measures; or 2) modestly poor scores (defined as −1 SD to −2 SD) on multiple memory measures relative to premorbid intellect. For example, one participant in this study had a WRAT-4 of 120, RBANS DMI of 117, HVLT-R Delayed Recall of 91 (almost −2 SDs below premorbid intellect), and BVMT-R Delayed Recall of 95 (almost −2 SDs below premorbid intellect).
Novel upper extremity motor task
To test for practice effects in this study, we used a novel upper extremity motor task that 1) has been used previously for studying motor learning in older adults (Schaefer, 2015; Schaefer, Dibble, & Duff, 2015; Schaefer & Duff, 2015; Schaefer, Patterson, & Lang, 2013); 2) has concurrent and ecological validity (Schaefer & Hengge, 2016); 3) is similar to other tasks yielding motor practice effects (Yan & Dick, 2006); and 4) is derived from standardized clinical assessments of hand function (Jebsen, Taylor, Trieschmann, Trotter, & Howard, 1969).
The motor task apparatus is illustrated in Figure 1A. One trial of the motor task was comprised of five repetitions to three different targets placed radially around a constant start location at a distance of 16 cm; thus, each trial equaled 15 repetitions total. The start location and all three targets were cups that were 9.5 cm in diameter and 5.8 cm in height. For each repetition, participants used their nondominant hand to acquire and transport two raw kidney beans from the start location to one of the three target locations with a conventional plastic spoon. At the start of each trial, participants’ first repetition was out to the ipsilateral target cup, next to the center target cup, and then to the contralateral cup, relative to the hand used. As noted above, participants repeated this sequence 5 times to complete the trial. Each trial began when the participants picked up the spoon and ended when the last two beans were dropped into the final cup, yielding a ‘trial time’ as the measure of performance. Additional details regarding the justification, design, and validity of this task have been published previously (Schaefer & Hengge, 2016; Schaefer & Lang, 2012). Importantly, performing this task with the nondominant hand is by design to ensure that the task is under-practiced and not over-learned, particularly in older adults (Schaefer, 2015), such that participants have the potential to show practice effects without confounds of floor or ceiling effects (Suchy, Kraybill, & Franchow, 2011). A modified Edinburgh Handedness Inventory was used to identify participants’ nondominant hand (Oldfield, 1971).
Figure 1.
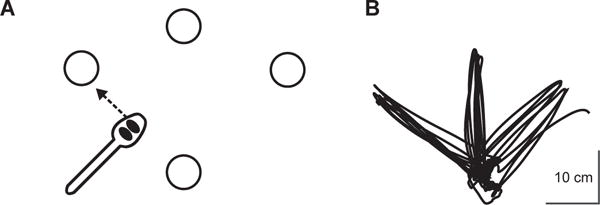
A) Overhead view of motor task apparatus. The start and center locations were placed at participants’ midlines while they were seated. Dimensions of all movement distances and target sizes are described in the Methods. B) Typical handpath over the course of one trial (i.e. five repetitions out and back to each of the three targets, equaling 15 repetitions per trial). Participants moved out and back first to the ipsilateral target, then to the center target, then to the contralateral target, and then back to the ipsilateral target again, and so on until the trial was over. Kinematic data were collected in a previous study using an electromagnetic six degree-of-freedom (6-DOF) movement recording system (Flock of Birds®, Ascension Technology Corp, Shelburne, VT) that was integrated into Motion Monitor software (Innovative Sports Training, Chicago, IL). Kinematic data were collected for purposes of paradigm validation in Schaefer & Hengge (2016), and are therefore only provided in this study for illustration.
As stated above, the measure of performance was the time taken to complete the 15 repetitions (i.e. “trial time”), with faster times indicating better performance, as participants were instructed to “move as quickly yet as accurately as possible.” All trials were timed to the nearest 100th of a second via stopwatch. Participants were allowed to adopt any specified pattern of upper extremity kinematics during training, thereby facilitating exploratory attempts for discovering successful movement strategies for completing the task (similar to Taubert et al., 2010). Figure 1B shows a typical handpath over the course of one trial (i.e. five movements out and back to each of the three targets), which has been published and validated previously (Schaefer & Duff, 2015; Schaefer & Hengge, 2016).
Participants first completed a baseline trial (i.e. 15 repetitions) of the motor task. They then completed nine additional trials for within-session practice (trials 2–10). This small dose of training, which generally takes 10–15 minutes to complete, is practical yet sufficient for yielding within-session improvements in performance that are related to longer-term acquisition and retention (Schaefer & Duff, 2015). Moreover, much higher doses of practice are tolerable by older populations with sensorimotor and cognitive impairments (Schaefer et al., 2015; Schaefer et al., 2013). Thus, this task was appropriate and safe for probing motor practice effects in this study. Participants were then re-tested a week later on a follow-up trial to identify any measureable practice effects across one week, an approached used previously with other neuropsychological assessments (Duff, 2014; Duff et al., 2008; Duff, Foster, & Hoffman, 2014; Duff et al., 2011).
Quantifying practice effects
We computed within-session practice effects by subtracting participants’ performance (measured in seconds, s) on the last practice trial from baseline, normalizing this difference to relative to baseline, and then expressing this as a percentage:
| (1) |
We have previously observed similar within-session effects on this motor task in non-demented older adults (Schaefer & Duff, 2015); thus, we aimed to replicate and potentially extend these findings in a clinical sample.
We also computed one-week practice effects in a similar manner, comparing participants’ performance on the one-week follow-up relative to their baseline performance:
| (2) |
In both measures, normalizing practice effects relative to baseline performance allowed us to account for any effects of frailty or relative slowness that may be present in upper extremity motor performance (Metter, Schrager, Ferrucci, & Talbot, 2005; Schaefer et al., 2015; Temprado et al., 2013; Toosizadeh, Mohler, & Najafi, 2015). In using this method for quantifying practice effects, positive values indicate better performance at the one-week follow-up; negative values indicate worse performance at the one-week follow-up. Similar measures (e.g. simple difference scores and ratios) have been used previously to document practice effects on other cognitive assessments (Duff, 2014; Duff et al., 2008; Duff, Chelune, & Dennett, 2012). These measures of practice effects were calculated once we evaluated test-retest correlations and confirmed significant differences between baseline and these trials of interests (see “Statistical analyses” below).
Statistical analyses
The SAS® statistical software program JMP® 10.0 (SAS Institute Inc., Cary, NC) was used for all statistical analysis (α = 0.05). We first calculated within- and between-session test-retest correlations (Pearson’s correlation coefficient, r) by comparing participants’ baseline performances to their performance on the next (2nd) trial and one week later, respectively. We next tested for any significant within-session improvement using a one-tailed paired t-test to compare the performance on the last (10th) trial relative to that of baseline. A paired t-test was similarly used to test for significant between-session improvements by comparing performances at one-week follow-up relative to baseline. The sizes of any significant effects were reported using η2 values.
We then calculated each participant’s within-session and one-week practice effects using Equations 1 and 2, respectively, and computed one-sided 95% confidence intervals for these values once assumptions of normality for the practice effect variable were confirmed with Shapiro-Wilk tests (within-session, W = 0.98; p = 0.62 and one-week, W = 0.98; p = 0.57). These analyses allowed us to test our primary hypothesis that older adults with Mild Cognitive Impairment would show measurable practice effects. To test our second hypothesis, we examined the correlation between our measures of practice effects on the motor task and demographic and clinical variables. The demographic variables included age, education, and gender. Clinical variables included an estimate of premorbid intellect (WRAT Reading subtest) depressive symptoms (GDS), and global cognition (RBANS Total Score Index). Significant Pearson’s correlation coefficients (r) greater than 0.59 were considered to be strong, between 0.30 and 0.59 were moderate, and below 0.30 were weak (Cohen, 1988).
We also ran more exploratory analyses to identify which specific cognitive domains tested by the RBANS were significantly related to the practice effects observed in this study. The RBANS Immediate Memory, Delayed Memory, Language, Attention, and Visuospatial/Constructional Indexes were included as measurable proxies for their respective cognitive domains. Using these RBANS Indexes as predictors, we ran separate backwards elimination stepwise regression analyses with the within-session and one-week practice effects as dependent variables (see Eqs. 1 and 2). All predictors were entered into the respective models, and only those that significantly contributed to the variance in the dependent variables were included in the final regression based on the criterion-to-remove of p > 0.05. Based on the results of the stepwise regression, raw trial time data were subjected to repeated-measures analyses of variance (ANOVAs) based on the median score of any significant RBANS Index(es) and the corresponding test-retest interval. Given the known limitations of retrospective stepwise regression in general, such as parameter bias, over-fitting, and an inflated probability of Type I errors (Copas, 1983), we validated our backwards stepwise regression using Mallow’s Cp criterion. Cp is an alternative measure of total squared error used to address overfitting concerns related to stepwise regression. Generally speaking, smaller Cp values indicate better model fits (Mallows, 1973). The strength of any significant effects here were reported using η2 values.
RESULTS
Participant characteristics
Of the 54 participants with amnestic Mild Cognitive Impairment, most were male (n = 28; 51.85% of total) and all were Caucasian. Summary statistics are provided in Table 1 for participants’ age, education, and other demographic and clinical variables. As noted in Table 1, the mean and SD for the RBANS Total Score Index was 86.06 ± 15.47 (range = 50 – 121). These clinical scores were comparable to earlier samples of participants with amnestic Mild Cognitive Impairment used for studying practice effects on cognitive tests (Duff et al., 2008; Duff et al., 2011). Individual RBANS Index scores are also provided in Table 1.
Table 1.
Participant characteristics.
| Mean ± SD | Median | Range | |
|---|---|---|---|
| Age | 75.8 ± 5.9 | 75 | 65–89 |
| Education | 16.6 ± 2.7 | 17 | 12–22 |
| WRAT-4 | 109.7 ± 8.0 | 109 | 93–126 |
| GDS | 3.4 ± 2.4 | 3.5 | 0–13 |
| HVLT-R Delayed Recall | 32.2 ± 12.9 | 32 | 19–60 |
| BVMT-R Delayed Recall | 33.5 ± 11.9 | 31 | 19–58 |
| RBANS Total Scale Index | 86.1 ± 15.5 | 86 | 50–121 |
| Attention Index | 99.5 ± 15.8 | 100 | 64–132 |
| Language Index | 91.1 ± 15.6 | 92 | 8–122 |
| Visuospatial/Constructional Index | 97.6 ± 17.5 | 100 | 62–136 |
| Immediate Memory Index | 82.2 ± 19.5 | 83 | 44–114 |
| Delayed Memory Index | 75.9 ± 24.5 | 78 | 40–117 |
N = 54; 28 males and 26 females.
WRAT-4 = Reading subtest of the Wide Range Achievement Test – 4, age-corrected.
GDS = 30-item Geriatric Depression Scale
HVLT-R = Delayed Recall age-corrected T-score, Hopkins Verbal Learning Test-Revised
BVMT-R = Delayed Recall age-corrected T-score, Brief Visuospatial Memory Test-Revised
RBANS = Repeatable Battery for the Assessment of Neuropsychological Status. Scores are age-normed, with a normal score of 100 and with a standard deviation of 15.
Test-retest correlations
The motor task selected for this study demonstrated strong test-retest correlations, such that participants’ baseline performance was significantly related to their performance on the second trial (r = 0.83; p < 0.0001) and one week later (r = 0.82; p < 0.0001).
Evidence of within- and between-session motor practice effects
Figure 2 illustrates the average responsiveness to practice immediately following the baseline trial. This performance curve within the initial session verified that the motor task used in this study was not over-learned, such that participants were not bound by ‘ceiling effects’ and were able to improve on the task with repeated exposure within a session. A paired one-tailed t-test indicated that participants were significantly faster on the last (10th) practice trial relative to baseline (t(106) = 4.59; p < 0.0001; η2 = 0.17). Figure 2 also shows that participants were faster at their one-week follow-up relative to baseline (t(106) = 1.78; p < 0.05; η2 = 0.03). Positive one-tailed confidence intervals provided additional evidence of within-session (95% CI [12.44, 19.76]) and one-week (95% CI [3.68, 10.96]) practice effects. Thus, these data supported our primary hypothesis that motor task improvements would occur with repeat testing within a brief practice session and one week later.
Figure 2.
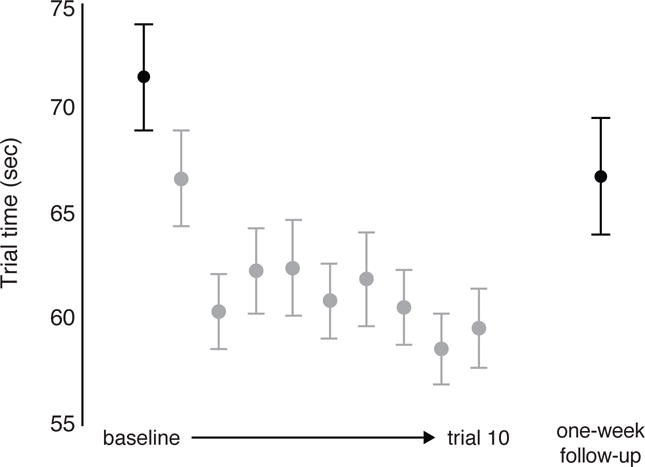
Mean ± SE trial times for baseline and one-week follow-up trials (shown in black for emphasis) and the nine remaining within-session practice trials during the initial session (shown in gray). Faster trial times indicate better performance.
No relationship between practice effects and demographic and clinical variables
There was no statistically significant relationship between our measures of practice effects and age (within-session, p = 0.99; one-week, p = 0.09), education (within-session, p = 0.27; one-week, p = 0.26), premorbid intellect on the WRAT Reading subtest (within-session, p = 0.30; one-week, p = 0.81), and depressive symptoms on the GDS (within-session, p = 0.28; one-week, p = 0.77). Males and females also did not differ in their practice effects after 10 trials (p = 0.27) or across one week (p = 0.92).
Relationship between practice effects and cognition
A second purpose of this study was to investigate if practice effects would be related to global cognition or other cognitive domains. Although we hypothesized that participants with lower cognitive scores would show smaller practice effects, least squares regression indicated no significant relationship between either practice effects measure and Total Scale score on the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (within-session, p = 0.16; one-week, p = 0.10). The Total Score on the RBANS is a composite score of global cognition based on participants’ performance on individual cognitive domains (i.e., indexes), thereby allowing us to further test whether the practice effects we observed in this study were related to specific cognitive domains rather than a composite of them all together. We therefore ran a backwards elimination stepwise regression for practice effects with each of the RBANS Index scores (Immediate Memory, Delayed Memory, Language, Attention, and Visuospatial/Constructional) and entered into the full model.
For within-session practice effects, all individual Index scores were eliminated based on p-values greater than 0.05 (all p ≥ 0.16), indicating that no single Index significantly predicted participants’ responsiveness to practice as measured by the amount of improvement over the 10 practice trials. For one-week practice effects, however, the final model determined that the only significant predictor of the extent of one-week practice effects on the motor task was the Delayed Memory Index (standardized β = −0.33; r = −0.33; adjusted r = −0.29; p = 0.01). Interestingly, lower Delayed Memory Index scores were associated with larger practice effects (Fig. 3), such that participants with worse delayed memory ability actually improved more from baseline to follow-up one week later. This was the case regardless of deficits in other cognitive domains such as language or immediate memory. Table 2 provides the iterative stepwise elimination of each predictor based on p-value for one-week practice effects, such that the least significant predictors were eliminated first (e.g. Language and Immediate Memory Index scores). The Visuospatial/Constructional Index was eliminated last as a potential predictor of such practice effects (p = 0.14). The Mallows Cp criteria suggested, however, that the model of best fit may include both the Delayed Memory Index and the Visuospatial/Constructional Index (Cp = 0.21) rather than just the Delayed Memory Index alone (Cp = 0.32) (Table 2).
Figure 3.
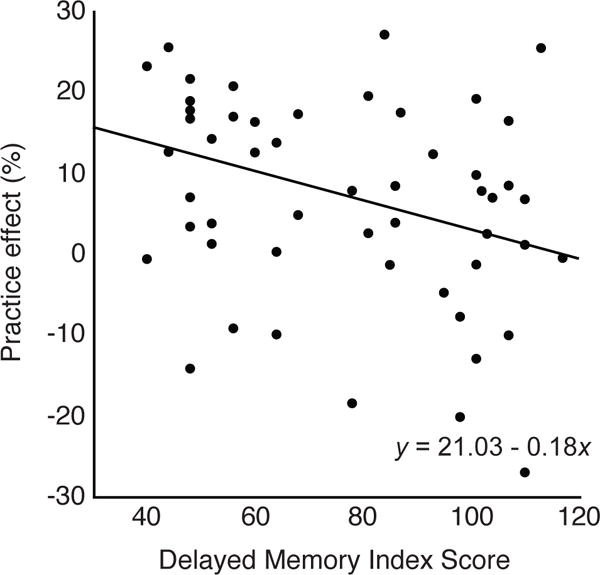
One-week practice effects are plotted for all participants as a function of RBANS Delayed Memory Index score. Linear predictor model equation is also shown for reference. Practice effects are expressed as a percentage of baseline performance (Eq. 2). Positive values along both the x- and y-axes indicate better scores.
Table 2.
Standardized regression coefficients (β), t-ratios, and p-values for each parameter, as well as R2 for the full and final models from backwards elimination stepwise regression. Cp values for each step are provided for validation purposes.
| Step | Parameter | □std β | t | p | Action | R2 | Cp |
|---|---|---|---|---|---|---|---|
| 1 | Full model | 0.15 | Entered | 0.15 | 6 | ||
| 2 | Language Index | −0.014 | −0.09 | 0.93 | Removed | 4.01 | |
| 3 | Immediate Memory Index | −0.046 | −0.20 | 0.84 | Removed | 2.05 | |
| 4 | Attention Index | −0.059 | −0.42 | 0.68 | Removed | 0.21 | |
| 5 | Visuospatial/Constructional Index | 0.19 | 1.49 | 0.14 | Removed | 0.32 | |
|
| |||||||
| Final model | |||||||
| Delayed Memory Index | −0.33 | −2.53 | 0.01 | 0.11 | |||
Dependent variable: One-week practice effect.
Cp = Mallow’s criterion, with smaller values indicated better model fit. Note that the values reported for each parameter are calculated after that parameter had been removed, e.g., Cp = 0.21 for Step 4 was calculated after the Attention Index score parameter was removed, leaving the Visuospatial/Constructional Index and Delayed Memory Index in the stepwise elimination.
Given the counter-intuitive association of larger one-week practice effects with poorer delayed memory, we grouped participants based on their Delayed Memory Index score for further analysis. The median score for this sample on the Delayed Memory Index of the RBANS was 78; thus, the 26 of 54 participants with index scores below 78 were in the bottom 50th percentile for delayed memory function, yet actually showed larger improvements on the motor task at the one-week follow-up relative to the 28 participants in the top 50th percentile (scores ≥78). Importantly, however, both groups had comparable mean ± SD performance at baseline (<78 = 74.11 ± 13.97 s vs. ≥78 = 73.02 ± 20.20 s). This interaction between timepoint (baseline vs. one-week follow-up; within-subject factor) and group (<78 vs. ≥78; between-subject factor) was statistically significant based on a 2×2 repeated-measures ANOVA of trial time (F(1, 52) = 5.57; p = 0.02; η2 = 0.008). Tukey HSD posthoc tests confirmed no significant difference between baseline performance between groups (p = 0.93), but the <78 group improved significantly at the one-week follow-up (p < 0.0001; η2 = 0.03); the ≥78 group showed no improvement one week later (p = 0.14). Even without the median split, when the Delayed Memory Index was modeled as a continuous rather than dichotomized variable, it was not a predictor of baseline performance (r = 0.07; p = 0.59). Thus, the differences in one-week practice effects observed between those with higher vs. lower delayed memory function were not due to differences in baseline proficiency (i.e., a ceiling effect without room for improvement) on the motor task itself.
DISCUSSION
The primary purpose of this study was to examine practice effects on a procedural memory motor task in older adults diagnosed with amnestic Mild Cognitive Impairment. Data from this study not only replicated previous findings of within-session motor practice effects in individuals with MCI (e.g., Rouleau et al., 2002), but also now demonstrate that these individuals have the capacity for procedurally-based effects one week later, regardless of their global cognitive score or any other demographic variable. These new findings appear to be in contrast to previous practice effects literature on declarative memory and other neuropsychological tasks (e.g., Calamia et al., 2012). Instead, the only significant predictor of practice effects was the Delayed Memory Index, with lower scores associated with larger motor practice effects.
While the negative relationship between delayed memory and motor practice effects might be counter-intuitive, they may actually be supported by recent evidence of compensatory interactions between the declarative and procedural memory systems. Individuals with fronto-striatal deficits due to Parkinson’s disease are known to have impaired procedural memory, yet behavioral and functional neuroimaging data have demonstrated that such deficits may be compensated for by intact declarative memory systems (see Roy, Park, Roy, & Almeida, 2015). For example, individuals with Parkinson’s disease can improve their motor sequence or skill learning through explicit memory strategies (Gobel, Blomeke, Zadikoff, Simuni, Weintraub, & Reber, 2013), and have increased a) prefrontal cortical activity during a nondeclarative habit-forming task and b) mediotemporal cortical activity in a weather-prediction task, relative to age-matched controls (Moody, Bookheimer, Vanek, & Knowlton, 2004). While there is debate about whether the declarative and procedural memory systems, when both intact, are cooperative or competitive (see Poldrack & Packard, 2003), there is emerging evidence that when one is impaired, the other may compensate (see also Roy & Park, 2010). This may in part explain how older adults with amnestic Mild Cognitive Impairment can show practice effects on motor tasks, be they over 24 hours (Dick et al., 1995; Hirono et al., 1997; Rouleau et al., 2002; Willingham, Peterson, Manning, & Brashear, 1997; Yan & Dick, 2006) or one week as in this study, despite significant explicit or declarative memory deficits. Although this study was not designed to directly test the compensatory interactions between the memory systems, our stepwise regression results are nevertheless consistent with previous work, and supports how such interactions could be exploited in rehabilitative interventions (Harrison, Son, Kim, & Whall, 2007; Machado et al., 2009; van Halteren-van Tilborg, Scherder, & Hulstijn, 2007).
Although visuospatial construction ability was eliminated from the stepwise regression based on a p-value criterion, other methods of model validation suggested it may have also been a predictor of motor practice effects, which is consistent with work identifying its role in procedural memory formation. For example, visuospatial working memory has been previously identified as a candidate mechanism underlying motor sequence learning (Anguera, Reuter-Lorenz, Willingham, & Seidler, 2010; Bo, Borza, & Seidler, 2009; Bo & Seidler, 2009; Schweighofer et al., 2011), which is thought to be a sub-process of skill acquisition (Willingham, 1998). Although these earlier studies typically excluded participants with lower global cognitive status based on the Mini-Mental Status Exam or the Montreal Cognitive Assessment, they suggest that individuals with clinical levels of visuospatial impairment would have impaired motor skill learning. This is in fact what is seen in neurological conditions that appear to selectively impair visuospatial function, such as Huntington’s disease (Heindel, Butters, & Salmon, 1988; Heindel, Salmon, Shults, Walicke, & Butters, 1989; Knopman & Nissen, 1991; Willingham, Koroshetz, & Peterson, 1996; see also Furtado & Mazurek, 1996) and Williams syndrome (Foti et al., 2013; Vicari, 2001; Vicari, Bellucci, & Carlesimo, 2001). Although the lowest Visuospatial/Constructional Index score in our sample was 62 (<1st percentile based on normative values), only 15 of the 54 participants were below one standard deviation from ‘normal’ on this index relative to age-matched normative data; thus, this study may not have been sufficiently powered to address whether visuospatial function independently predicted motor practice effects (pairwise correlation, r = 0.20; p = 0.15) or would reliably be included in the final regression equations. Future research is necessary to fully probe the extent to which visuospatial deficits impair procedural memory formation.
We further note limitations that are inherent to our sample and our design. We acknowledge that this sample’s relatively high level of education (>16 years on average) challenged our intent to investigate how this demographic variable influenced the observed motor practice effects. Although there were no significant effects of education on within-session or one-week motor practice effects, one cannot necessarily rule out the extent to which lower levels of education might influence test-retest characteristics on tasks involving procedural memory. We also did not capture ages below 65 years old in this study, further limiting our ability to test our secondary hypotheses related to demographics. In addition, we acknowledge that the RBANS does not include an index for executive functioning, which could be a missed domain of interest. Lastly and importantly, we point out that our final model yielded by the stepwise regression accounted for only 11% of the variance in our one-week practice effect measure (see Table 2); thus, there are a number of factors (executive function notwithstanding) that influence the extent to which patients with Mild Cognitive Impairment retain procedural memories, such as functional status (Siengsukon, Al-Dughmi, Al-Sharman, & Stevens, 2015) or medication status (McGaugh & Cahill, 1997; Soeter & Kindt, 2011). In general, one should use caution in interpreting the model(s) of best fit yielded by stepwise regression, as the process can be viewed as retrospective data mining that may yield different results depending on the algorithm used (forward or backward), the order of parameter entry/deletion, and the number of candidate parameters (Derksen & Keselman, 1992). With this in mind, future directions of this work include longitudinal and more inclusive studies that are designed to examine concurrent changes in cognitive and motor functioning in Mild Cognitive Impairment, and what might best predict such changes.
In short, this study demonstrated that individuals diagnosed with amnestic Mild Cognitive Impairment can show significant one-week practice effects on a novel motor task. This further supports that short-term practice effects that draw upon procedural memory may persist even when other practice effects that draw upon declarative memory are attenuated or nonexistent.
Acknowledgments
This work was supported by the National Institute on Aging of the National Institutes of Health under grant numbers K01AG047926 and R01AG045163. The authors also acknowledge Taylor Atkinson, Bonnie Dalley, and Kayla Suhrie for their assistance in data collection.
Footnotes
The authors have no financial interests or other conflicts of interest to disclose.
References
- Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Contributions of spatial working memory to visuomotor learning. Journal of Cognitive Neuroscience. 2010;22(9):1917–1930. doi: 10.1162/jocn.2009.21351. [DOI] [PubMed] [Google Scholar]
- Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Failure to engage spatial working memory contributes to age-related declines in visuomotor learning. Journal of Cognitive Neuroscience. 2011;23(1):11–25. doi: 10.1162/jocn.2010.21451. [DOI] [PubMed] [Google Scholar]
- Bo J, Borza V, Seidler RD. Age-related declines in visuospatial working memory correlate with deficits in explicit motor sequence learning. Journal of Neurophysiology. 2009;102:2744–2754. doi: 10.1152/jn.00393.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo J, Seidler RD. Visuospatial working memory capacity predicts the organization of acquired explicit motor sequences. Journal of Neurophysiology. 2009;101:3116–3125. doi: 10.1152/jn.00006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Robertson EM, Press DZ. Sequence skill acquisition and off-line learning in normal aging. PLoS One. 2009;4:e6683. doi: 10.1371/journal.pone.0006683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamia M, Markon K, Tranel D. Scoring higher the second time around: meta-analyses of practice effects in neuropsychological assessment. The Clinical Neuropsychologist. 2012;26:543–570. doi: 10.1080/13854046.2012.680913. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Cooper DB, Epker M, Lacritz L, Weine M, Rosenberg RN, Honig L, Cullum CM. Effects of practice on category fluency in Alzheimer’s disease. The Clinical Neuropsychologist. 2001;15(1):125–128. doi: 10.1076/clin.15.1.125.1914. [DOI] [PubMed] [Google Scholar]
- Copas JB. Regression, Prediction and Shrinkage. Journal of the Royal Statistical Society. Series B (Methodological) 1983;45(3):311–335. [Google Scholar]
- Darby D, Maruff P, Collie A, McStephen M. Mild cognitive impairment can be detected by multiple assessments in a single day. Neurology. 2002;59:1042–1046. doi: 10.1212/wnl.59.7.1042. [DOI] [PubMed] [Google Scholar]
- Derksen S, Keselman HJ. Backward, forward and stepwise automated subset selection algorithms: frequency of obtaining authentic and noise variables. British Journal of Mathematical and Statistical Psychology. 1992;45:265–282. [Google Scholar]
- Dick MB, Nielson KA, Beth RE, Shankle WR, Cotman CW. Acquisition and long-term retention of a fine motor skill in Alzheimer’s disease. Brain and Cognition. 1995;29:294–306. doi: 10.1006/brcg.1995.1283. [DOI] [PubMed] [Google Scholar]
- Duff K. One-week practice effects in older adults: tools for assessing cognitive change. The Clinical Neuropsychologist. 2014;28:714–725. doi: 10.1080/13854046.2014.920923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Beglinger LJ, Schultz SK, Moser DJ, McCaffrey RJ, Haase RF, Paulsen JS. Practice effects in the prediction of long-term cognitive outcome in three patient samples: a novel prognostic index. Archives of Clinical Neuropsychology. 2007;22(1):15–24. doi: 10.1016/j.acn.2006.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Beglinger LJ, Van Der Heiden S, Moser DJ, Arndt S, Schultz SK, Paulsen JS. Short-term practice effects in amnestic mild cognitive impairment: implications for diagnosis and treatment. International Psychogeriatrics. 2008;20:986–999. doi: 10.1017/S1041610208007254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Callister C, Dennett K, Tometich D. Practice effects: a unique cognitive variable. The Clinical Neuropsychologist. 2012;26:1117–1127. doi: 10.1080/13854046.2012.722685. [DOI] [PubMed] [Google Scholar]
- Duff K, Chelune G, Dennett K. Within-session practice effects in patients referred for suspected dementia. Dementia and Geriatric Cognitive Disorders. 2012;33:245–249. doi: 10.1159/000339268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Foster NL, Hoffman JM. Practice effects and amyloid deposition: preliminary data on a method for enriching samples in clinical trials. Alzheimer Disease and Associated Disorders. 2014;28:247–252. doi: 10.1097/WAD.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Hobson VL, Beglinger LJ, O’Bryant SE. Diagnostic Accuracy of the RBANS in Mild Cognitive Impairment: Limitations on Assessing Milder Impairments. Archives of Clinical Neuropsychology. 2010;25:429–41. doi: 10.1093/arclin/acq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Lyketsos CG, Beglinger LJ, Chelune G, Moser DJ, Arndt S, McCaffrey RJ. Practice effects predict cognitive outcome in amnestic mild cognitive impairment. The American Journal of Geriatric Psychiatry. 2011;19:932–939. doi: 10.1097/JGP.0b013e318209dd3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti F, Menghini D, Mandolesi L, Federico F, Vicari S, Petrosini L. Learning by observation: insights from Williams syndrome. PLoS One. 2013;8(1):e53782. doi: 10.1371/journal.pone.0053782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado JC, Mazurek MF. Behavioral characterization of quinolinate-induced lesions of the medial striatum: relevance for Huntington’s disease. Experimental Neurology. 1996;138(1):158–168. doi: 10.1006/exnr.1996.0054. [DOI] [PubMed] [Google Scholar]
- Gobel EW, Parrish TB, Reber PJ. Neural correlates of skill acquisition: decreased cortical activity during a serial interception sequence learning task. Neuroimage. 2011;58:1150–1157. doi: 10.1016/j.neuroimage.2011.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobel EW, Blomeke K, Zadikoff C, Simuni T, Weintraub S, Reber PJ. Implicit perceptual-motor skill learning in Mild Cognitive Impairment and Parkinson’s disease. Neuropsychology. 2013;27(3):314–321. doi: 10.1037/a0032305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BE, Son GR, Kim J, Whall AL. Preserved implicit memory in dementia: a potential model for care. American Journal of Alzheimer’s Disease and Other Dementias. 2007;22(4):286–293. doi: 10.1177/1533317507303761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel WC, Butters N, Salmon DP. Impaired learning of a motor skill in patients with Huntington’s disease. Behavioral Neuroscience. 1988;102(1):141–147. doi: 10.1037//0735-7044.102.1.141. [DOI] [PubMed] [Google Scholar]
- Heindel WC, Salmon DP, Shults CW, Walicke PA, Butters N. Neuropsychological evidence for multiple implicit memory systems: a comparison of Alzheimer’s, Huntington’s, and Parkinson’s disease patients. The Journal of Neuroscience. 1989;9(2):582–587. doi: 10.1523/JNEUROSCI.09-02-00582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono N, Mori E, Ikejiri Y, Imamura T, Shimomura T, Ikeda M, Yamadori A. Procedural memory in patients with mild Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 1997;8(4):210–216. doi: 10.1159/000106633. [DOI] [PubMed] [Google Scholar]
- Howard JH, Howard DV, Dennis NA, Kelly AJ. Implicit learning of predictive relationships in three-element visual sequences by young and old adults. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34(5):1139–1157. doi: 10.1037/a0012797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs DH, Adair JC, Williamson DJ, Na DL, Gold M, Foundas AL, Shuren JE, Heilman KM. Apraxia and motor-skill acquisition in Alzheimer’s disease are dissociable. Neuropsychologia. 1999;37(7):875–80. doi: 10.1016/s0028-3932(98)00139-0. [DOI] [PubMed] [Google Scholar]
- Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Archives of Physical Medicine and Rehabilitation. 1969;50:311–319. [PubMed] [Google Scholar]
- Knopman D, Nissen MJ. Procedural learning is impaired in Huntington’s disease: evidence from the serial reaction time task. Neuropsychologia. 1991;29:245–254. doi: 10.1016/0028-3932(91)90085-m. [DOI] [PubMed] [Google Scholar]
- Machado S, Cunha M, Minc D, Portella CE, Velasques B, Basile LF, Ribeiro P. Alzheimer’s disease and implicit memory. Arquivos de Neuro-psiquiatria. 2009;67(2A):334–342. doi: 10.1590/s0004-282x2009000200034. [DOI] [PubMed] [Google Scholar]
- Mallows CL. Some Comments on Cp. Technometrics. 1973;15:661–675. [Google Scholar]
- McGaugh JL, Cahill L. Interaction of neuromodulatory systems in modulating memory storage. Behavioural Brain Research. 1997;83(1–2):31–38. doi: 10.1016/s0166-4328(97)86042-1. [DOI] [PubMed] [Google Scholar]
- Metter EJ, Schrager M, Ferrucci L, Talbot LA. Evaluation of movement speed and reaction time as predictors of all-cause mortality in men. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2005;60:840–846. doi: 10.1093/gerona/60.7.840. [DOI] [PubMed] [Google Scholar]
- Moody TD, Bookheimer SY, Vanek Z, Knowlton BJ. An implicit learning task activates medial temporal lobe in patients with Parkinson’s disease. Behavioral Neuroscience. 2004;108:438–442. doi: 10.1037/0735-7044.118.2.438. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status. San Antonio, TX: The Psychological Coporation; 1998. [Google Scholar]
- Rapport LJ, Axelrod BN, Theisen ME, Brines DB, Kalechstein AD, Ricker JH. Relationship of IQ to verbal learning and memory: test and retest. Journal of Clinical and Experimental Neuropsychology. 1997;19:655–666. doi: 10.1080/01688639708403751. [DOI] [PubMed] [Google Scholar]
- Rouleau I, Salmon DP, Vrbancic M. Learning, retention and generalization of a mirror tracing skill in Alzheimer’s Disease. Journal of Clinical and Experimental Neuropsychology. 2002;24(2):239–250. doi: 10.1076/jcen.24.2.239.997. [DOI] [PubMed] [Google Scholar]
- Roy S, Park NW. Dissociating the memory systems mediating complex tool knowledge and skills. Neuropsychologia. 2010;48(10):3026–3036. doi: 10.1016/j.neuropsychologia.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Roy S, Park NW, Roy EA, Almeida QJ. Interaction of memory systems during acquisition of tool knowledge and skills in Parkinson’s disease. Neuropsychologia. 2015;66:55–66. doi: 10.1016/j.neuropsychologia.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Schaefer SY. Preserved motor asymmetry in late adulthood: is measuring chronological age enough? Neuroscience. 2015;294:51–59. doi: 10.1016/j.neuroscience.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Schaefer SY, Dibble LE, Duff K. Efficacy and feasibility of functional upper extremity task-specific training for older adults with and without cognitive impairment. Neurorehabilitation and Neural Repair. 2015;29:636–644. doi: 10.1177/1545968314558604. [DOI] [PubMed] [Google Scholar]
- Schaefer SY, Duff K. Rapid Responsiveness to Practice Predicts Longer-Term Retention of Upper Extremity Motor Skill in Non-Demented Older Adults. Frontiers in Aging Neuroscience. 2015;7:214. doi: 10.3389/fnagi.2015.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Hengge CR. Testing the concurrent validity of a naturalistic upper extremity reaching task. Experimental Brain Research. 2016;234(1):229–240. doi: 10.1007/s00221-015-4454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Lang CE. Using dual tasks to test immediate transfer of training between naturalistic movements: A proof-of-principle study. Journal of Motor Behavior. 2012;44:313–327. doi: 10.1080/00222895.2012.708367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Patterson CB, Lang CE. Transfer of training between distinct motor tasks after stroke: implications for task-specific approaches to upper-extremity neurorehabilitation. Neurorehabilitation and Neural Repair. 2013;27:602–612. doi: 10.1177/1545968313481279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer N, Lee JY, Goh HT, Choi Y, Kim SS, Stewart JC, Winstein CJ. Mechanisms of the contextual interference effect in individuals poststroke. Journal of Neurophysiology. 2011;106:2632–2641. doi: 10.1152/jn.00399.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siengsukon C, Al-Dughmi M, Al-Sharman A, Stevens S. Sleep Parameters, Functional Status, and Time Post-Stroke are Associated with Offline Motor Skill Learning in People with Chronic Stroke. Frontiers in Neurology. 2015;6:225. doi: 10.3389/fneur.2015.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Disrupting reconsolidation: pharmacological and behavioral manipulations. Learning & Memory. 2011;18:357–366. doi: 10.1101/lm.2148511. [DOI] [PubMed] [Google Scholar]
- Suchy Y, Kraybill ML, Franchow E. Practice effect and beyond: reaction to novelty as an independent predictor of cognitive decline among older adults. Journal of the International Neuropsychological Society. 2011;17(1):101–111. doi: 10.1017/S135561771000130X. [DOI] [PubMed] [Google Scholar]
- Taubert M, Draganski B, Anwander A, Muller K, Horstmann A, Villringer A, Ragert P. Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. The Journal of Neuroscience. 2010;30:11670–11677. doi: 10.1523/JNEUROSCI.2567-10.2010. doi: 30/35/11670 [pii] 10.1523/JNEUROSCI.2567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temprado JJ, Sleimen-Malkoun R, Lemaire P, Rey-Robert B, Retornaz F, Berton E. Aging of sensorimotor processes: a systematic study in Fitts’ task. Experimental Brain Research. 2013;228(1):105–116. doi: 10.1007/s00221-013-3542-0. [DOI] [PubMed] [Google Scholar]
- Toosizadeh N, Mohler J, Najafi B. Assessing Upper Extremity Motion: An Innovative Method to Identify Frailty. Journal of the American Geriatrics Society. 2015;63:1181–1186. doi: 10.1111/jgs.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Halteren-van Tilborg IA, Scherder EJ, Hulstijn W. Motor-skill learning in Alzheimer’s disease: a review with an eye to the clinical practice. Neuropsychology Review. 2007;17:203–212. doi: 10.1007/s11065-007-9030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari S. Implicit versus explicit memory function in children with Down and Williams syndrome. Down’s Syndrome, Research and Practice. 2001;7(1):35–40. doi: 10.3104/reports.112. [DOI] [PubMed] [Google Scholar]
- Vicari S, Bellucci S, Carlesimo GA. Procedural learning deficit in children with Williams syndrome. Neuropsychologia. 2001;39:665–677. doi: 10.1016/s0028-3932(01)00012-4. [DOI] [PubMed] [Google Scholar]
- Willingham DB. A neuropsychological theory of motor skill learning. Psychological Review. 1998;105:558–584. doi: 10.1037/0033-295x.105.3.558. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Koroshetz WJ, Peterson EW. Motor skills have diverse neural bases: Spared and impaired skill acquisition in Huntington’s Disease. Neuropsychology. 1996;10:315–321. [Google Scholar]
- Willingham DB, Peterson EW, Manning C, Brashear HR. Patients with Alzheimer’s disease who cannot perform some motor skills show normal learning of other motor skills. Neuropsychology. 1997;11:261–271. doi: 10.1037//0894-4105.11.2.261. [DOI] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Petersen RC. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Yan JH, Dick MB. Practice effects on motor control in healthy seniors and patients with mild cognitive impairment and Alzheimer’s disease. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition. 2006;13(3–4):385–410. doi: 10.1080/138255890969609. [DOI] [PubMed] [Google Scholar]


