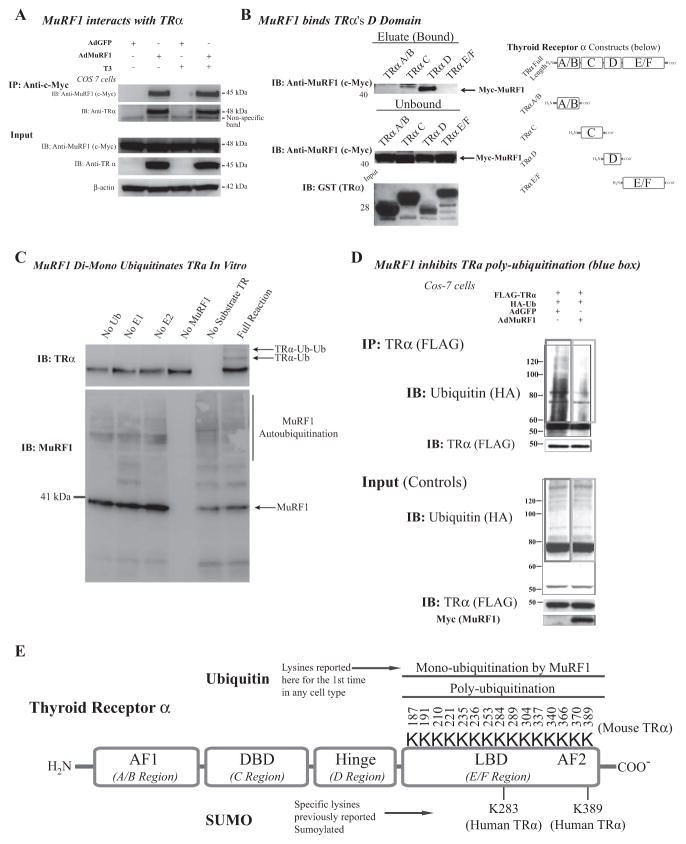
Figure 5.
MuRF1 interacts with and mono-ubiquitinates TRα. (A) Cos-7 cells transduced with AdMuRF1 and treated with 16.6 nM T3 for 2 h were used in analysis. Whole cell lysates were immunoprecipitated with myc-MuRF1 and probed for endogenous TRα to evaluate protein—protein interaction as shown in the blot (left). (B) GST-tagged recombinant peptides for each TR domain were used in an immunoprecipitation assay with myc-MuRF1 followed by immunoblot to establish the specific domain bound by MuRF1 (right). Blots shown on top right show the MuRF1-bound regions of TR (predominantly D); the top blot shows the bound myc-MuRF1 (eluate) for each domain construct. The middle blot shows unbound myc-MuRF1 and the bottom membrane is a Ponceau stain of each isolated peptide to confirm expression of the expected molecular weight protein (constructs were also sequenced to verify product, data not shown). At the far right is a diagram indicating the various domains, for reference. (C) An in vitro biochemical assay was used to assess ubiquitination with exogenous MuRF1 and was detected by immunoblot with HA-tagged Ub antibody. (D) Cos-7 cells co-transfected with HA-Ub and FLAG-TRα and transduced with AdMuRF1 (or AdGFP) at MOI 25 for 4 h; whole cell lysates were submitted to immunoprecipitation of FLAG-TRα followed by immunoblot for HA-Ub. The red box highlights ubiquitination in controls, whereas the blue box demonstrates the decrease in ubiquitination when MuRF1 expression is increased. Empty vector control (FLAG and HA) experiment blots are shown in left panel. (E) Diagram of TRα with known protein domains indicated in addition to the lysines within the E/F (ligand binding) domain with the corresponding (murine) amino acid sequence position (total of 15 residues). This region includes two lysines known to be targeted for regulation in the human isoform of TR for SUMOylation (K283 and K389).