Scheme 2. Synthesis of GC-1a and GC-1b Reagents and conditions.
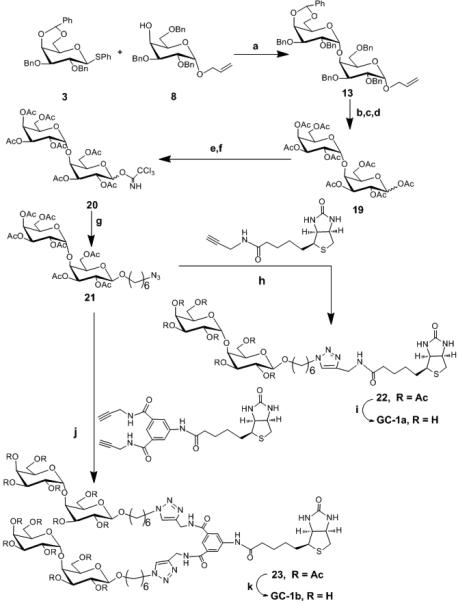
a) CH2Cl2, AgOTf, p-NO2PhSCl, TTBP, −78°C, 60% b) PdCl2, NaOAc, AcOH, H2O, rt, 54% c) Pd(OH)2, H2, EtOAc / EtOH, rt , 67%, d) Ac2O, pyridine, DMAP, 0°C to rt , 57% e) H2NNH2.HOAc, THF, rt, 63% f) K2CO3, CH2Cl2, Cl3CCN, rt, 77% g) CH2Cl2, HO(CH2)6Cl, TMSOTf, −30°C to rt, 55% h) CuSO4.5H2O, C6H7O6Na, THF/H2O, 70% i) MeOH, NaOMe, rt, quantitative j) CuSO4.5H2O, C6H7O6Na, THF/H2O, 70% k) MeOH, NaOMe, rt quantitative.
