Abstract
Chromosome lesions which could be interpreted as “fragile sites” on the distal end of the long arm of the X chromosome were identified during a cytogenetic study of 160 mentally retarded adult males with no apparent cause of their mental retardation and one normal adult female with a family history of fra (X) syndrome. Peripheral blood samples were cultured in either M199 or RPMI 1640 medium with FUdR or BrdU. Metaphases were examined for chromosome lesions or fragile sites on the distal end of Xq and 3 distinct sites were observed: Xq26, Xq27.2, and Xq27.3. Other chromosome lesions at Xq28 were observed and interpreted as nonspecific telomeric structural changes. Chromosome lesions were observed in cells from 14 of the 161 individuals. These included: 5 patients with an Xq26 site, 2 with the recently reported Xq27.2 site, 4 with the Xq27.3 site (characteristic of the fra (X) syndrome), 2 with nonspecific telomeric structural changes, and one individual with 2 lesions (a nonspecific telomeric structural change and an Xq26 site). Additional research is necessary to determine the frequency and clinical significance, if any, of lesions occurring in this region of the X chromosome and to distinguish among heritable fragile sites, constitutive fragile sites, and nonspecific telomeric structural changes.
Keywords: common and heritable fragile sites, fragile X or Martin-Bell syndrome, prenatal diagnosis, Xq26, Xq27.2, and Xq27.3 sites, nonspecific telomeric structural changes
INTRODUCTION
The fragile X [fra (X)] or Martin-Bell syndrome is a common cause of mental retardation and is characterized by the presence of an Xq27.3 fragile site (gene symbol FRAXA) at the distal end of the long arm of the X chromosome [Hagerman and McBogg, 1983; Krawczun et al., 1985; Chudley and Hagerman, 1987]. Expression of the Xq27.3 site in 4% of male cells and in 2% of female cells grown in folate-deficient culture conditions is considered diagnostic for the fra (X) syndrome. Fragile sites are observed as breaks or gaps in chromosomes when cells are grown in specific culture conditions. Fragile sites are classified as either common or heritable and expression may depend on the culture medium. Common or constitutive sites are prevalent and may be expressed homozygously and seen in all individuals [Sutherland and Hecht, 1985]. Heritable, or rare, sites are expressed as a nonstaining gap at an exact position, inherited in a Mendelian codominant manner, and may be induced by specific culture conditions [Sutherland and Hecht, 1985].
Studies of common fragile sites using aphidicolin [Glover et al., 1984; Schlegelberger et al., 1989] and caffeine [Yunis and Soreng, 1984] have shown the existence of sites on the X chromosome including Xp22.3 (gene symbol FRAXB) and Xq22 (gene symbol FRAXC) but no common fragile sites were reported on the distal end of Xq. However, a common fragile site at Xq27 was reported recently in normal males with no family history of X-linked mental retardation [Ledbetter et al., 1986]. Additionally, Kähkönen [1988] observed an Xq27 site in one or 2 cells in every 50 cells analyzed in a survey of both normal and mentally retarded individuals. Recently, a common site at Xq27.2 (gene symbol FRAXD) different from the rare Xq27.3 site seen in fra (X) syndrome patients was reported and may explain the Xq27 site seen in normal individuals or individuals without the fra (X) syndrome [Ledbetter and Ledbetter, 1988; Ramos et al., 1989; Sutherland and Baker, 1989]. A possible fragile site at Xq26 was also reported in metaphases from a severely retarded male and his normal mother [Bühler et al., 1982]. This site has been implicated in chromosome rearrangements seen in cancer cells [Sutherland and Hecht, 1985].
Nonspecific telomeric structural changes that resemble fragile sites have been reported [Steinbach et al., 1982; Jenkins et al., 1986] and may also account for the Xq27 fragile sites reported previously in normal control individuals. Nonspecific telomeric structural changes occur on virtually all chromosomes, have a nonrandom distribution, resemble the fra (X) site morphologically, and may have similar origins to fragile sites [Steinbach et al., 1982; Jenkins et al., 1986]. In view of recent cytogenetic information on fragile sites and nonspecific telomeric structural changes on the distal long arm of the X chromosome, we report our cytogenetic experience with 161 adult individuals who were analyzed for the fra (X) syndrome.
MATERIALS AND METHODS
Patients and Cytogenetic Methods
The patients in this study included 160 mentally retarded adult males ranging in age from 18 to 86 years with no recognizable cause of their mental retardation and a 27-year-old female with normal intelligence but with the fra (X) syndrome diagnosed in her sister’s son. Peripheral blood samples (0.5 ml) were cultured in triplicate by using medium 199 (M199), RPMI 1640 medium supplemented with fluorodeoxyuridine (FUdR), and RPMI 1640 medium supplemented with bromodeoxyuridine (BrdU). M199 is a folate-deficient medium which allows expression of folate-sensitive fragile sites. FUdR, a thymidylate synthetase inhibitor, promotes expression of these sites, and BrdU, a thymidine analog, induces a separate class of fragile sites [Sutherland and Hecht, 1985].
Peripheral blood cultures were prepared by using M199 supplemented with 5% fetal calf serum, phytohemagglutinin, and antibiotics with the pH adjusted to 7.8. The cultures were incubated at 37°C for 96 hours and colcemid (0.15 μg/ml) added 45 minutes prior to harvest.
Peripheral blood samples were also cultured by using RPMI 1640 medium, supplemented by 15% fetal calf serum, phytohemagglutinin, and antibiotics. The cultures were incubated for 96 hours at 37°C with FUdR (10−7M) added 24 hours prior to harvest and colcemid (0.15 μg/ml) added 45 minutes before harvest.
Peripheral blood samples were cultured by using RPMI 1640 medium supplemented with 5% fetal calf serum, phytohemagglutinin, and antibiotics. Incubation was for 72 hours at 37°C and BrdU (30 mg/L) was added 6 hours before harvest. Colcemid (0.15 μg/ml) was added 45 minutes prior to harvest.
Cells were harvested following treatment with 0.56% KCl hypotonic solution for 8 minutes at 37°C. Cells were fixed with 3:1 methanol/acetic acid and washed four times. Chromosome slides were air dried by using conventional methods and stained with Giemsa. A minimum of 125 cells (e.g., 50 from M199, 25 from RPMI 1640 and FUdR, 50 from RPMI 1640 and BrdU) were analyzed for chromosome lesions from each individual. Additional cells (e.g., 100 cells) were analyzed if a chromosome lesion was observed in the distal long arm of the X chromosome. Chromosome gaps and breaks resembling fragile sites were recorded and distinguished by their appearance and location from nonspecific telomeric structural changes as described by Steinbach et al. [1982] and Jenkins et al. [1986] after destaining and banding with the GTG method.
RESULTS
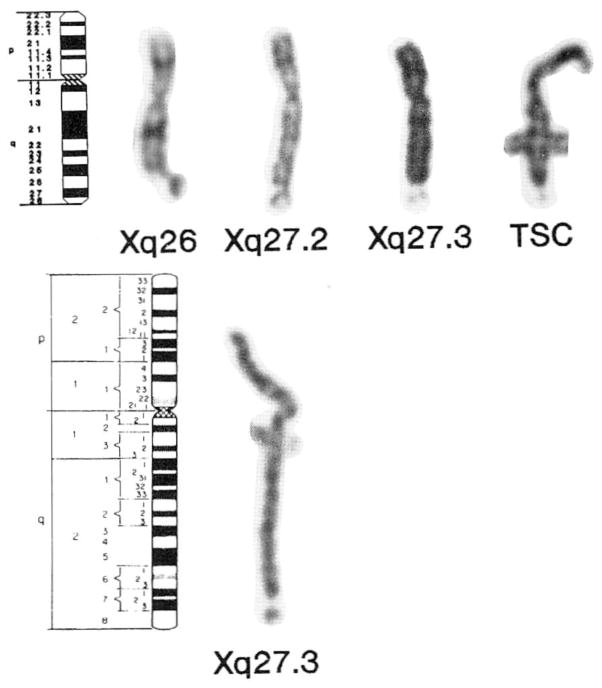
Four different sites at the distal end of the long arm of the X chromosome were observed in 14 of the 161 adult individuals. These sites were: Xq26, Xq27.2, Xq27.3 (observed in patients with the fra (X) syndrome), and Xq28 lesions classified as nonspecific telomeric structural changes (Fig. 1). Table I summarizes cytogenetic data from the 14 patients with chromosome breaks or gaps resembling fragile sites and nonspecific telomeric structural changes at the distal end of Xq. The Xq26 site, previously reported in one family [Bühler et al., 1982], was identified in 5 of our individuals; 4 patients with the Xq27.3 site (characteristic for the fra (X) syndrome), 2 individuals with the recently described Xq27.2 site, 2 patients with Xq28 sites interpreted as nonspecific telomeric structural changes, and one individual (patient E) with 2 Xq sites: Xq26 and a nonspecific telomeric structural change (Table I).
Fig. 1.
Collection of X chromosomes showing lesions at Xq26, Xq27.2, Xq27.3, and nonspecific telomeric structural changes (TSC). The idiogram (400 band level) shows the location of the bands for the X chromosome. The break at Xq26 clearly occurs proximal to the Giemsa-positive Xq27 band. The Xq27.2 site is proximal to and distinct from the Xq27.3 band observed in fra (X) syndrome patients. A nonspecific telomeric structural change, atypical in appearance for a fragile site, is located at Xq28 or qterminus (the Xp22 fragile site can also be seen in this X chromosome) [top]. X chromosome idiogram (850 band level) and a representative prometaphase X chromosome from a patient with fra (X) syndrome banded with the GTG method showing the location of the Xq27.3 fragile site [bottom].
TABLE I.
Chromosome “Fragile Sites” Observed on Distal Xq in 14 of 161 Individuals Screened for the Fragile X Syndrome
| Patient | Age (yr) | Sex | Culture Medium/supplement | No. cells counted | Fragile site (No. observed) |
|---|---|---|---|---|---|
| A | 47 | M | M199/ | 100 | Xq26 (1) |
| RPMI 1640/FUdR | 50 | — | |||
| RPMI 1640/BrdU | 50 | — | |||
| B | 27 | M | M199/ | 50 | Xq26 (1) |
| RPMI 1640/FUdR | 25 | — | |||
| RPMI 1640/BrdU | 50 | — | |||
| C | 50 | M | M199/ | 50 | — |
| RPMI 1640/FUdR | 63 | Xq26 (1) | |||
| RPMI 1640/BrdU | 50 | — | |||
| D | 40 | M | M199/ | 50 | — |
| RPMI 1640/FUdR | 25 | Xq26 (1) | |||
| RPMI 1640/BrdU | 50 | — | |||
| E | 27 | F | M199/ | 68 | — |
| RPMI 1640/FUdR | 132 | Xq26 (1) TSCa (1) |
|||
| F | 44 | M | M199/ | 50 | — |
| RPMI 1640/FUdR | 25 | Xq26 (1) | |||
| RPMI 1640/BrdU | 50 | — | |||
| G | 46 | M | M199/ | 50 | — |
| RPMI 1640/FUdR | 25 | — | |||
| RPMI 1640/BrdU | 50 | Xq27.2 (1) | |||
| H | 76 | M | M199/ | 50 | — |
| RPMI 1640/FUdR | 94 | — | |||
| RPMI 1640/BrdU | 200 | Xq27.2 (1) | |||
| I | 42 | M | M199/ | 153 | TSCa (1) |
| RPMI 1640/FUdR | 79 | — | |||
| J | 41 | M | M199/ | 30 | — |
| RPMI 1640/FUdR | 122 | TSCa (1) | |||
| K | 28 | M | M199/ | 50 | Xq27.3 (13) |
| RPMI 1640/FUdR | 25 | Xq27.3 (12) | |||
| RPMI 1640/BrdU | 50 | — | |||
| L | 75 | M | M199/ | 85 | — |
| RPMI 1640/FUdR | 50 | Xq27.3 (9) | |||
| RPMI 1640/BrdU | 50 | — | |||
| M | 47 | M | M199/ | 50 | Xq27.3 (18) |
| RPMI 1640/FUdR | 25 | Xq27.3 (15) | |||
| RPMI 1640/BrdU | 50 | — | |||
| N | 37 | M | M199/ | 50 | Xq27.3 (23) |
| RPMI 1640/FUdR | 25 | Xq27.3 (9) | |||
| RPMI 1640/BrdU | 50 | — |
Nonspecific telomeric structural changes at Xq28.
DISCUSSION
Our survey with a relatively large number of patients is the first attempt to identify and characterize previously recognized sites (e.g., Xq27.3), as well as recently described fragile sites (e.g., Xq27.2), and nonspecific telomeric structural changes that could mask as qterminus fragile sites. We have shown that multiple chromosome lesions that could be interpreted as fragile sites do occur in about 10% of patients analyzed for the fra (X) syndrome. These sites included: Xq26, Xq27.2, Xq27.3, and other chromosome lesions interpreted as nonspecific telomeric structural changes. Special attention must be given not only to the location of the chromosome lesion but also to its appearance before the lesion is classified as a fragile site or a nonspecific telomeric structural change. Better guidelines should be established for the classification of a fragile site at the distal Xq region (e.g., Xq27.3), which has clinical significance, or a nonspecific telomeric structural change with no known significance.
Although there is a paucity of Xq26 sites reported in the literature [Bühler et al., 1982], we observed this site in our laboratory in cells grown in both M199 and RPMI 1640 medium with FUdR from 6 different patients. The Xq26 site has also been implicated as a cause of chromosome rearrangements in cancer cells [Sutherland and Hecht, 1985] and x-ray-induced chromosome breakage at this site has also been reported [Jonasson and Holmberg, 1973]. This site is clearly distinct and proximal to the Xq27.2 or Xq27.3 site seen in several of our patients.
The Xq27.2 site was seen in our study in 2 mentally retarded adult males without the classical features of fra (X) syndrome or family history of mental retardation and is clearly distinct and proximal to the Xq27.3 site characteristic of patients with the fra (X) syndrome. Interestingly, the Xq27.2 site was expressed in RPMI 1640 medium with BrdU and not in M199 or RPMI 1640 medium with FUdR from our 2 patients.
Clearly, the fragile site associated with the fra (X) syndrome is not the only chromosome lesion observed in cells from an individual, as evidenced by our patient E (Table I) who had both the Xq26 and a second lesion classified as a nonspecific telomeric structural change at Xq28. Although there are subtle but distinct differences among the three distal Xq chromosome sites (Xq26, Xq27.2, Xq27.3) observed in our study, special care with banded chromosomes is needed to accurately identify the location of these sites as well as to distinguish these fragile sites from nonspecific telomeric chromosome changes. Since as little as 2% expression may be considered diagnostic in females for the fra (X) syndrome, the presence of a fragile site diagnostic for this condition vs. a nonspecific telomeric structural change in close proximity to the Xq27.3 fragile site makes the cytogenetic interpretation difficult. An incorrect diagnosis based on cytogenetic analysis alone could be particularly devastating for prenatal diagnosis or in genetic counseling of family members.
In summary, we identified: the Xq27.3 site (characteristic of the fra (X) syndrome) in 4 individuals, 2 clearly distinct fragile sites proximal to Xq27.3 (e.g., Xq26, Xq27.2) in 8 patients, and nonspecific telomeric structural changes at Xq28 in 3 patients from our cytogenetic survey of 161 adult patients with mental retardation or a family history of fra (X) syndrome. Although the frequencies of Xq26, Xq27.2, or nonspecific telomeric structural changes were low in our patients, the potential did exist for a diagnostic error by misinterpreting these chromosome lesions as Xq27.3 sites. For example, in several of our patients chromosome lesions were observed in the first 50 cells and the diagnosis of fra(X) syndrome may have been made in these individuals if the chromosome lesion was classified as an Xq27.3 site and if additional cells were not analyzed. The cytogeneticist must be aware of all chromosome lesions including nonspecific telomeric structural changes that may occur in the Xq26–28 region. In view of the rapidly expanding cytogenetic information on heritable fragile sites, constitutive fragile sites, and nonspecific telomeric structural changes, we recommend that each laboratory record all chromosome lesions, particularly on the distal long arm, and this information should be accumulated on control individuals as well as patients evaluated for the fra (X) syndrome. This cytogenetic experience is required before the location and identification of fragile sites are determined and to distinguish between fragile sites and nonspecific telomeric structural changes.
Acknowledgments
We thank the Tennessee Department of Mental Health and Mental Retardation for their financial support. We also thank the staff and patients at the Clover Bottom Developmental Center, Nashville, Tennessee.
References
- Bühler EM, Hadziselimovic F, Pira U. A variant of the fra(X) syndrome. Hum Genet. 1982;61:273–275. doi: 10.1007/BF00296460. [DOI] [PubMed] [Google Scholar]
- Chudley AE, Hagerman RJ. Fragile X Syndrome. J Pediatr. 1987;110:821–831. doi: 10.1016/s0022-3476(87)80392-x. [DOI] [PubMed] [Google Scholar]
- Glover TW, Berger C, Coyle J, Echo B. DNA polymerase a inhibition by aphidicolin induces gaps and breaks at common fragile sites in human chromosomes. Hum Genet. 1984;67:136–142. doi: 10.1007/BF00272988. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, McBogg PM. The Fragile X Syndrome, Diagnosis Biochemistry, Intervention. Dillon, Colorado: Spectra Publishing Co., Inc; 1983. [Google Scholar]
- Jenkins EC, Brown WT, Brooks J, Duncan CJ, Sanz MM, Silverman WP, Kusum PL, Masia A, Katz E, Lubin RA, Nolin SL. Low frequencies of apparently fragile X chromosomes in normal control cultures: A possible explanation. Exp Cell Biol. 1986;54:40–48. doi: 10.1159/000163342. [DOI] [PubMed] [Google Scholar]
- Jonasson J, Holmberg M. Evidence for an inverse relationship between x-ray induced chromatid and chromosome breakage in human chromosomes. Hereditas. 1973;75:259–266. doi: 10.1111/j.1601-5223.1973.tb01167.x. [DOI] [PubMed] [Google Scholar]
- Kähkönen M. Population cytogenetics of folate-sensitive fragile sites. I. Common fragile sites. Hum Genet. 1988;80:344–348. doi: 10.1007/BF00273649. [DOI] [PubMed] [Google Scholar]
- Krawczun MS, Jenkins EC, Brown WT. Analysis of the fragile-X chromosome: localization and detection of the fragile site in high resolution preparations. Hum Genet. 1985;69:209–211. doi: 10.1007/BF00293026. [DOI] [PubMed] [Google Scholar]
- Ledbetter SA, Ledbetter DH. A common fragile site at Xq27: Theoretical and practical implications. Am J Hum Genet. 1988;42:694–702. [PMC free article] [PubMed] [Google Scholar]
- Ledbetter DH, Ledbetter SA, Nussbaum RL. Implications of fragile X expression in normal males for the nature of the mutation. Nature. 1986;324:161–163. doi: 10.1038/324161a0. [DOI] [PubMed] [Google Scholar]
- Ramos FJ, Emanuel BS, Spinner NB. Expression of a common fragile site at Xq27.2 under conditions of thymidylate stress in patients referred for fragile-X analysis and controls. Am J Hum Genet. 1989;45:A105. [Google Scholar]
- Schlegelberger B, Gripp K, Grote W. Common fragile sites in couples with recurrent spontaneous abortions. Am J Med Genet. 1989;32:45–51. doi: 10.1002/ajmg.1320320111. [DOI] [PubMed] [Google Scholar]
- Steinbach P, Barbi G, Böler T. On the frequency of telomeric structural changes induced by culture conditions suitable for fragile X expression. Hum Genet. 1982;61:160–162. doi: 10.1007/BF00274209. [DOI] [PubMed] [Google Scholar]
- Sutherland GR, Baker E. The common fragile site (FRAXD) is at Xq27.2 and can be distinguished from the fragile X (FRAXA) at Xq27.3. Cytogenet Cell Genet. 1989;51:1086–1087. [Google Scholar]
- Sutherland GR, Hecht F. Oxford Monographs on Medical Genetics. Vol. 13. Oxford: Oxford University Press; 1985. Fragile sites on human chromosomes. [Google Scholar]
- Yunis JJ, Soreng AL. Constitutive fragile sites and cancer. Science. 1984;225:1199–1204. doi: 10.1126/science.6239375. [DOI] [PubMed] [Google Scholar]