Scheme 2.
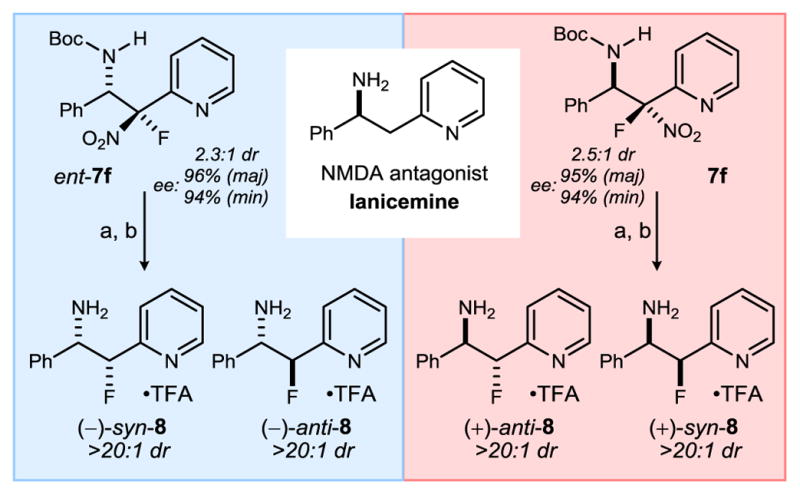
Lanicemine (AZD6765) and Preparation of All Four Stereoisomers of ‘β-Fluoro-Lanicemine’ (8).
Reaction conditions: a) Bu3SnH (4 equiv.), AIBN (0.4 equiv), benzene, 80 °C, 180 min (dr ≈ 2:1 anti:syn, 74%); b) TFA, CH2Cl2, 180 min (98%). In each case, diastereomers 8 were purified by reverse phase preparatory HPLC as their TFA salt adducts (see SI). Enantiomeric excess (ee) for stereoisomers 8 follow from ee of 7f/ent-7f.
