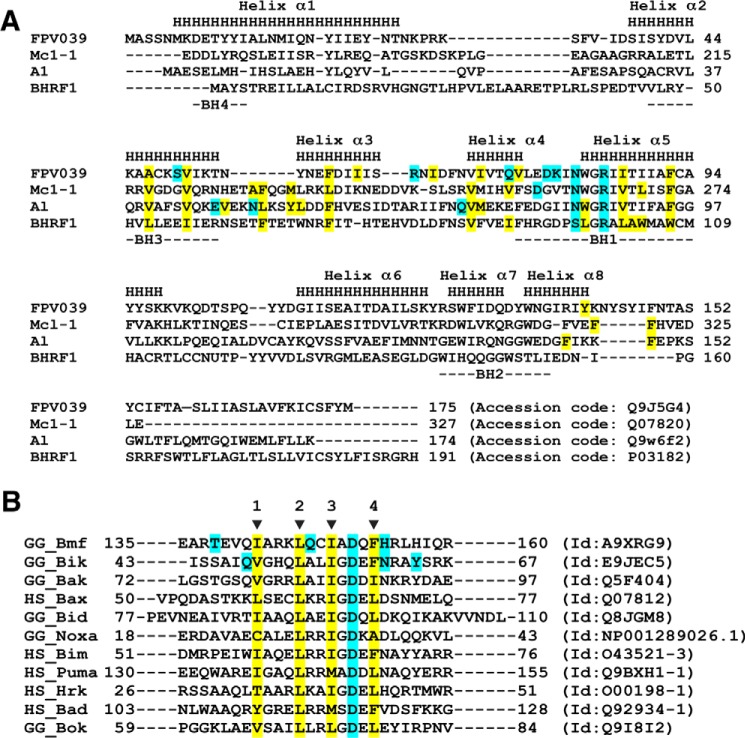
Figure 5.
Sequence alignments of FPV039 with Bcl-2 family members. A, structure-based sequence alignment of FPV039 with related cellular Bcl-2 proteins Mcl-1 and A1 as well as the closest viral Bcl-2 protein BHRF1. The α-helical secondary structure elements indicated (denoted as H and labeled Helices 1–8) are based on FPV039. BH motifs 1–4 are marked underneath the aligned sequences. Hydrophobic residues involved in the interactions with BH3 domain peptides are highlighted in yellow, and residues that are involved in hydrogen bonds or ionic interactions are highlighted in cyan. Accession numbers are as follows: FPV039, Q9J5G4; Mcl-1, Q07820; A1, Q9W6F2; and BHRF1, P03182. B, sequence alignment of BH3 domains used in affinity measurements. The four conserved hydrophobic residues in the BH3 motif are indicated by arrows and numbered. The hydrophobic residues involved in hydrophobic interactions with FPV039 in the case of Bmf and Bik BH3 domains are highlighted in yellow. Residues that form polar contacts with FPV039 are highlighted in cyan for Bmf and Bik BH3 domains as is the conserved aspartic acid of the BH3 domains. Sequence accession numbers are shown next to each sequence.