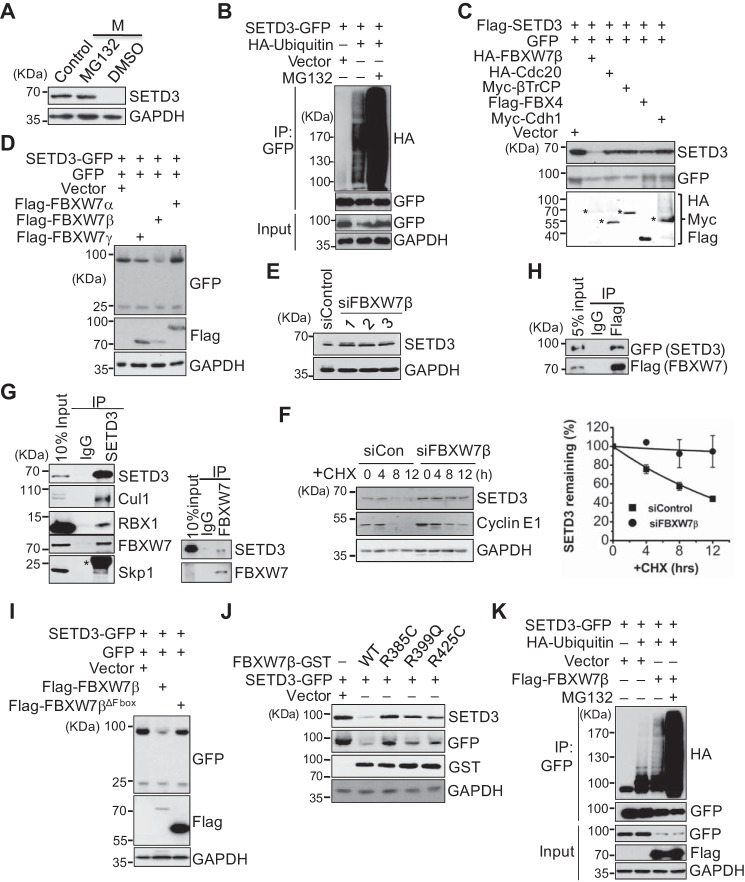
Figure 2.
SETD3 is degraded in a SCFFBXW7β- and proteasome-dependent manner. A, proteasome-dependent degradation of SETD3. Cells were synchronized in M phase and then treated with DMSO or 20 μm MG132 for 5 h before harvesting. Protein levels were analyzed by Western blotting. Asynchronous cells served as a control. B, in vivo ubiquitination assays were performed using 293T cells co-transfected with SETD3-GFP and an empty vector or HA-ubiquitin with or without MG132 treatment. SETD3 was immunoprecipitated with a GFP antibody, and the ubiquitinated levels were detected by immunoblots with α-HA. Cell lysates (Input) served as a control. C, several E3 ligases were co-transfected with FLAG-SETD3 and GFP in 293T cells, and protein levels of SETD3 were examined. GFP levels served as a control. The expressions of the indicated E3 ligases were detected by immunoblotting. Asterisks represent the corresponding proteins of expressed E3 ligases. D, three isoforms of FBXW7 were individually co-transfected with SETD3-GFP and the GFP vector. Relative protein levels of SETD3 were examined. E, knockdown of FBXW7 increased endogenous SETD3 protein levels. HeLa cells were transfected with control siRNA or three validated siRNAs targeting FBXW7β. Endogenous SETD3 levels were analyzed by Western blotting with GAPDH as a loading control. F, cells transfected with siControl or siFBXW7β RNA were treated with CHX. SETD3 stability was monitored at the indicated time points. The levels of cyclin E1 were used as a control (left panel). Two independent experiments were quantified by densitometry of Western blot analysis using ImageJ and presented as mean ± S.D. (right panel). G, SETD3 interacts with subunits of the SCFFBXW7β complex examined by reciprocal co-IP experiments. Co-IP assays were performed using SETD3 or FBXW7 antibody, and the subunits of the SCFFBXW7β complex, including Cul1, Rbx1, FBXW7, and Skp1 (left panel), or SETD3 (right panel) were analyzed by Western blotting. Ten percent of the total cell lysates (Input) and nonspecific IgG IP served as a control. Asterisk represents the light chain of IgG. H, co-IP assay was performed using M2 FLAG resin in 293T cells expressing SETD3-GFP and FLAG-FBXW7β. The interaction between SETD3 and FBXW7β was analyzed by Western blotting. Five percent of total cell lysates (5% input) were loaded as a control. I, F-box domain of FBXW7β is required for SETD3 degradation. Empty vector, wild-type, or F-box deletion mutant of FBXW7β was co-transfected with SETD3-GFP and the GFP vector, and the protein levels of SETD3 were examined. J, pathological mutants of FBXW7β construct derived from somatic mutations showed reduced ability to destruct SETD3. Cells expressing SETD3-GFP were co-transfected with empty vector, WT, or FBXW7β-GST mutants. Exogenous SETD3 levels in the indicated cells were analyzed by Western blotting probed with α-GFP and α-SETD3 antibodies. K, 293T cells were transfected with an empty vector, SETD3-GFP, HA-ubiquitin, or FLAG-FBXW7β as indicated, followed by IP with α-GFP. The ubiquitinated SETD3 was detected by Western blotting with α-HA.