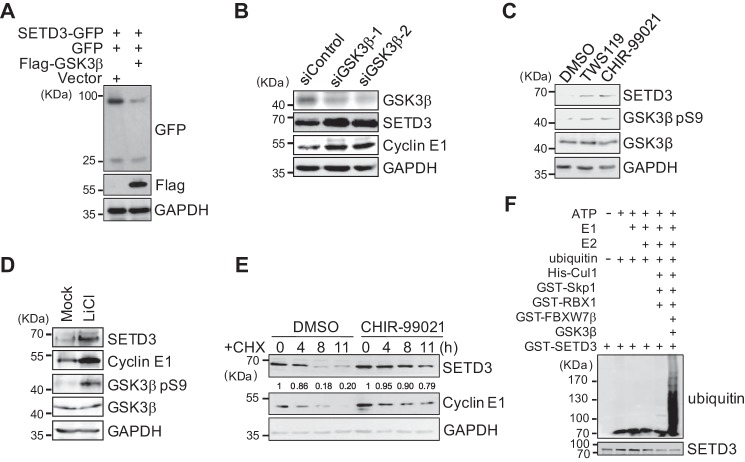
Figure 3.
SETD3 is degraded in a GSK3β-dependent manner. A, GFP vector and SETD3-GFP construct were co-transfected with an empty vector or FLAG-GSK3β into 293T cells. Relative levels of SETD3-GFP were analyzed by Western blotting. B, knockdown of GSK3β increased endogenous SETD3 levels. The 293T cells were transfected with control siRNA or two validated siRNAs targeting GSK3β. Protein levels of SETD3 and cyclin E1 were examined. C and D, inhibition of GSK3β increased SETD3 levels. HeLa cells were treated with different doses of the GSK3β inhibitor for 48 h in C or with 40 mm LiCl for 48 h in D. The endogenous SETD3 levels were examined by Western blotting. The cyclin E1 levels served as a control. The levels of phosphorylated GSK3β at Ser-9 were used to indicate the inhibitory efficiency of GSK3β. E, cells were treated with DMSO or a GSK3β inhibitor PL-02-061 in the presence of CHX. SETD3 stability was monitored at the indicated time points. F, in vitro ubiquitination assays were performed using individual recombinant proteins incubated with recombinant GST-SETD3, and the polyubiquitinated SETD3 bands were immunoblotted with an α-ubiquitin antibody.