Figure 9.
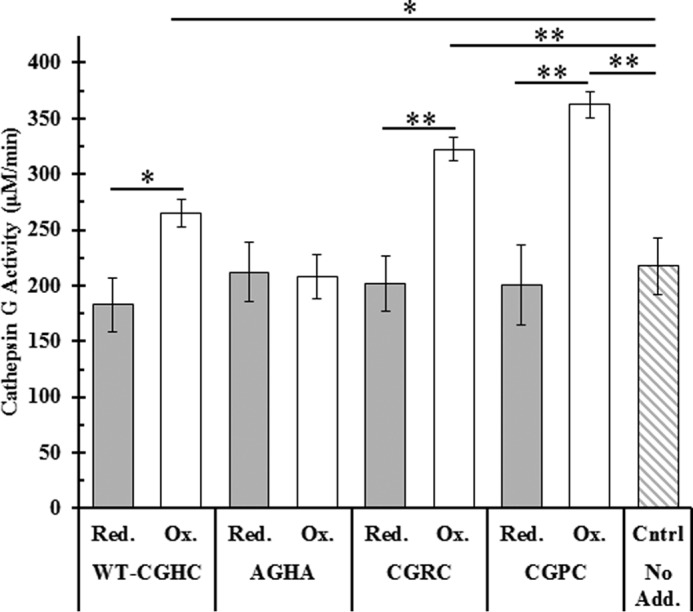
Effect of the oxidation state of PDI on cathepsin G activity in washed platelet releasate. Washed platelets were first incubated with either prereduced (gray) or preoxidized PDI (white) and then activated with 0.1 unit/ml thrombin. Platelet releasates were clarified with centrifugation and assayed for cathepsin G activity using a colorimetric probe monitored at 410 nm. Cathepsin G activities are shown as averages of triplicate experiments; error bars represent S.E. Paired t tests were used to determine significance: *, p < 0.05; **, p < 0.01. Red., reduced; Ox., oxidized; Cntrl, control; No Add., no additions.
