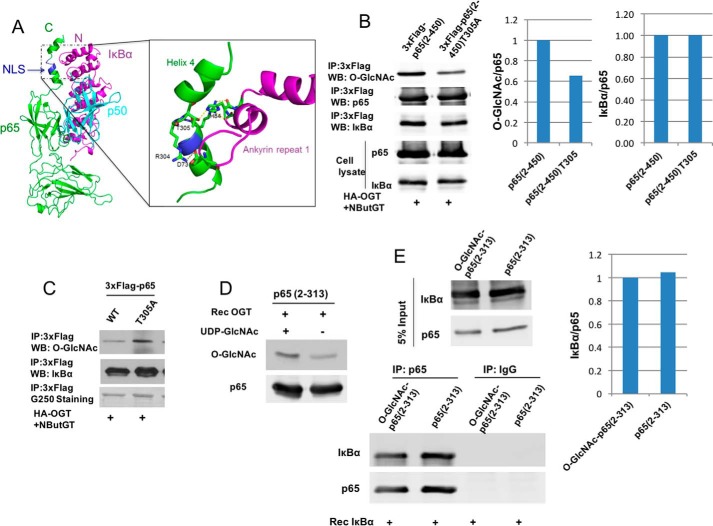
Figure 4.
O-GlcNAcylation of p65 at Thr-305 does not affect the binding of p65 to IκBα. A, crystal structure of p65/p50 heterodimer binding to IκBα (Protein Data Bank code 1NFI). Green, p65; cyan, p50; magenta, IκBα. Blue, p65 nuclear localization signal (NLS). The structure in the box shows the interactions between NLS of p65 and ankyrin repeat 1 of IκBα. Thr-305 sits right after the nuclear localization signal of p65 between helix 3 and helix 4 and forms a hydrogen bond with His-84 on IκBα. B, HEK 293T cells were cotransfected with plasmids expressing 3xFLAG-tagged p65(2–450) or T305A and the HA-OGT plasmid and then incubated overnight with NButGT. FLAG-tagged p65 was immunoprecipitated, and O-GlcNAc and p65-associated IκBα were detected by immunoblotting and quantified. Whole-cell lysates were also subjected to Western blot analysis using the indicated antibodies. C, HEK 293T cells were cotransfected with plasmids expressing 3xFLAG-tagged p65 or T305A and the HA-OGT plasmid and then incubated overnight with NButGT. FLAG-tagged p65 was immunoprecipitated, and O-GlcNAc and p65-associated IκBα were detected by immunoblotting. D, recombinant p65 fragment 2–313 prepared from bacteria was O-GlcNAcylated with Rec OGT in vitro. O-GlcNAcylated p65 was detected by Western blot. E, modified or unmodified p65 from D was incubated with recombinant IκBα for 2 h at 4 °C. p65 was immunoprecipitated, and associated IκBα was detected by immunoblotting and quantified. The experiments were repeated twice with similar results.