Figure 6.
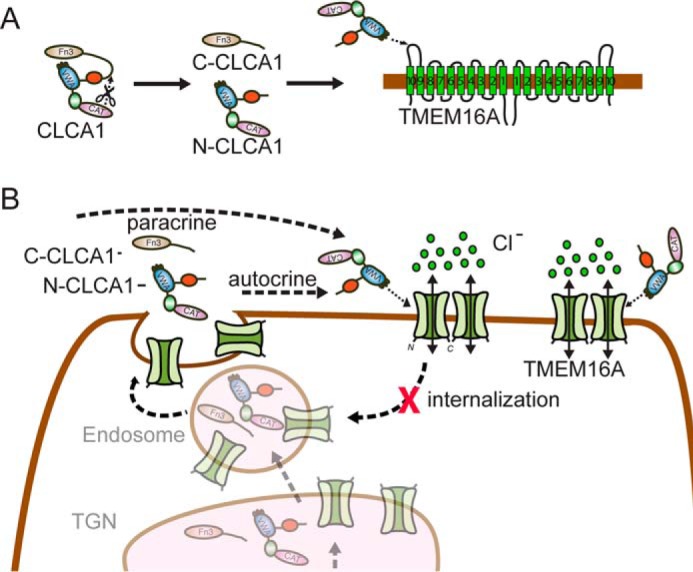
Model for CLCA1-mediated activation of TMEM16A. A, in full-length CLCA1, the VWA domain within the N-terminal region of the protein (N-CLCA1) is unable to engage TMEM16A, possibly because of a self-interaction with the FnIII domain in the C-terminal portion (C-CLCA1) that masks the VWA. Following self-cleavage, N-CLCA1 and C-CLCA1 likely dissociate, allowing the N-CLCA1 VWA domain to directly engage TMEM16A, likely via the α9-α10 loop of the channel. B, N-CLCA1 secreted by the same cell (autocrine) or by adjacent cells (paracrine) binds and stabilizes TMEM16A at the cell surface, likely by preventing its recycling, thereby increasing TMEM16A surface expression and ICaCC density.
