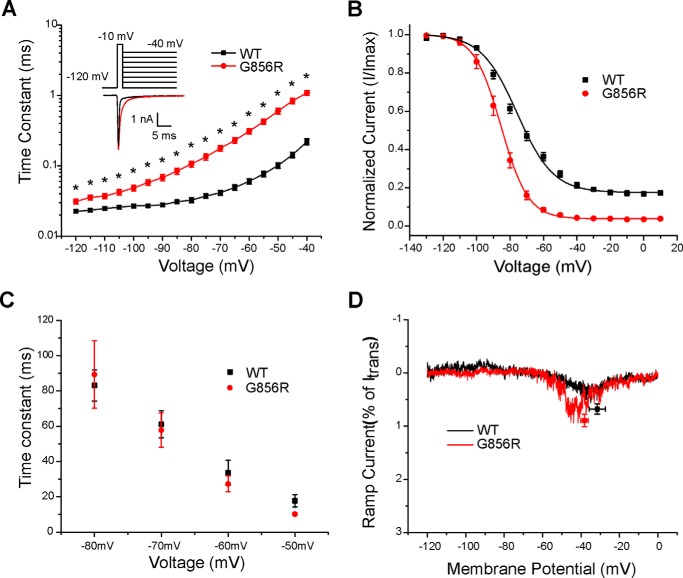
Figure 3.
Deactivation is slowed by the G856R mutation. A, deactivation time constants for WT and G856R channels. Tail currents were elicited by a brief 0.5-ms depolarization to −10 mV followed by a series of hyperpolarizing pulses ranging from −120 to −40 mV from a holding potential of −120 mV. Current decay of the tail current was fitted with a single exponential equation and plotted as a function of the hyperpolarizing step. G856R channels (red circles; n = 15) deactivate significantly slower than WT channels (black squares; n = 20). Inset, superimposed tail current traces of WT (black) and G856R (red) channels at −40 mV. B, comparison of steady-state slow inactivation for WT (black squares; n = 12) and G856R (red circles; n = 11) channels. Slow inactivation was assessed with 30-s prepulses that varied from −130 to +10 mV, followed by a brief hyperpolarization (−120 mV for 100 ms) to remove fast inactivation and a depolarizing test pulse to −10 mV to determine the fraction of available current. Peak inward currents were normalized and plotted as a function of the prepulse membrane potential and fit with a Boltzmann function. C, similar time constants for development of closed-state inactivation between WT (black squares) and G856R (red circles) channels. D, representative ramp currents in response to slow depolarizations (0.2 mV/ms) that were normalized to the peak current acquired during the activation protocol and plotted as a function of membrane potential for WT (black squares) and G856R (red circles) channels.