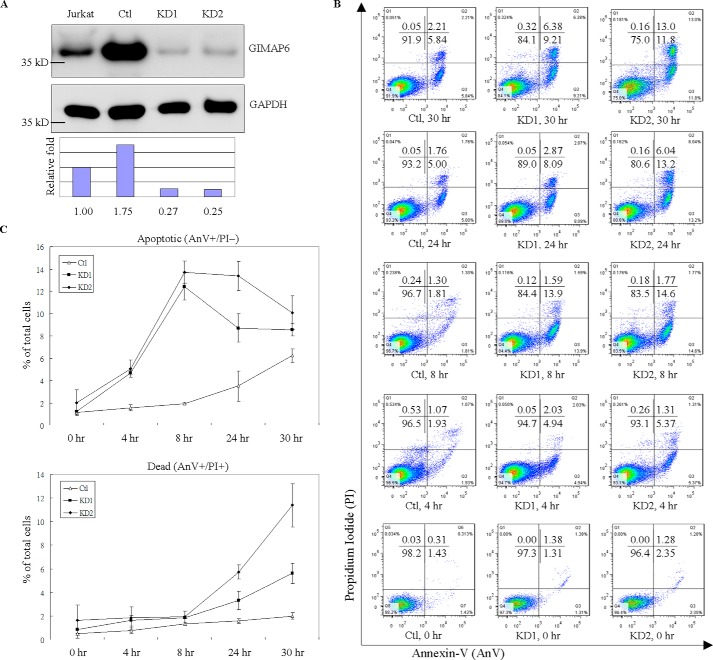
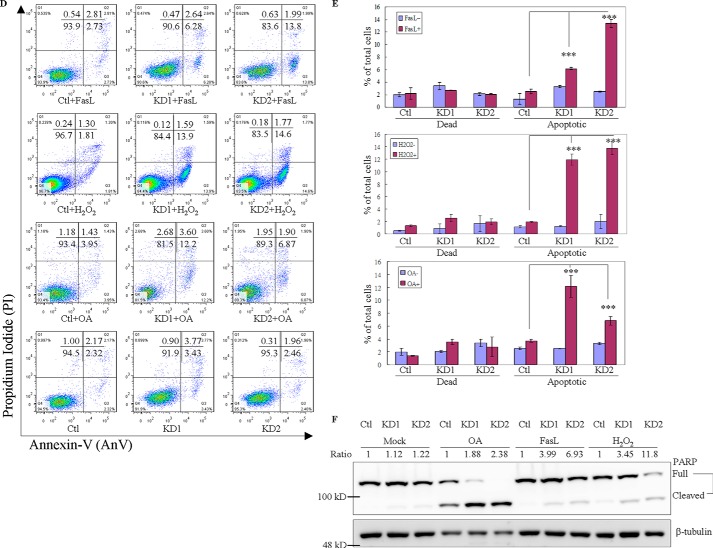
Figure 2.
GIMAP6 functions as an anti-apoptotic effector in Jurkat T lymphocytes. A, reduction of GIMAP6 protein in Jurkat T lymphocytes by anti-GIMAP6 shRNA knockdown was confirmed by immunoblot analysis. The Ctl expressed a relatively higher level of GIMAP6 compared with the two knockdown lines (KD1 and KD2) or even compared with the parental Jurkat T cell (Jurkat). The relative -fold expression levels were calculated as GIMAP6/GAPDH ratio, with the relative signal intensity in the Jurkat lane taken as 1. B, knockdown of GIMAP6 accelerated cell apoptosis/death in a H2O2-mediated multiple time point apoptosis assay. Jurkat-derived cell lines (Ctl, KD1, and KD2) were treated with 100 μm H2O2 for 4, 8, 24, and 30 h to induce cell apoptosis/death. Cells were then harvested and stained with Annexin V-FITC/PI, followed by flow cytometry analysis (Fig. 2B). The quantitative results are shown in C. Cells that stained Annexin-V+/PI− were considered to be apoptotic cells, whereas cells that were Annexin-V+/PI+ were considered to be dead cells. The data represent the average of two independent experiments with three triplicate measurements (mean ± S.D.). D, the effects of GIMAP6 on H2O2-mediated, FasL-mediated, and OA-mediated apoptosis. Jurkat-derived cell lines were treated with either 100 μm H2O2 for 8 h, 8 ng/ml FasL for 3 h, or 50 nm OA for 6 h to induce cell apoptosis. Cells were then harvested and stained with Annexin V-FITC/PI, followed by flow cytometry analysis. The quantified results are shown in E. The results represent the average of at least two independent experiments with triplicate measurements (mean ± S.D.; ***, p < 0.001, Student's t test). F, similarly, the various cell lines were treated with apoptosis-inducing agents for 24 h and then analyzed by immunoblot analysis to identify the full-length and cleaved form of PARP. The PARP cleavage ratio was calculated as cleaved/(cleaved + full-length) PARP and normalized against β-tubulin. Relative signal intensity (Ratio) in the Ctl lane for each condition was taken as 1.