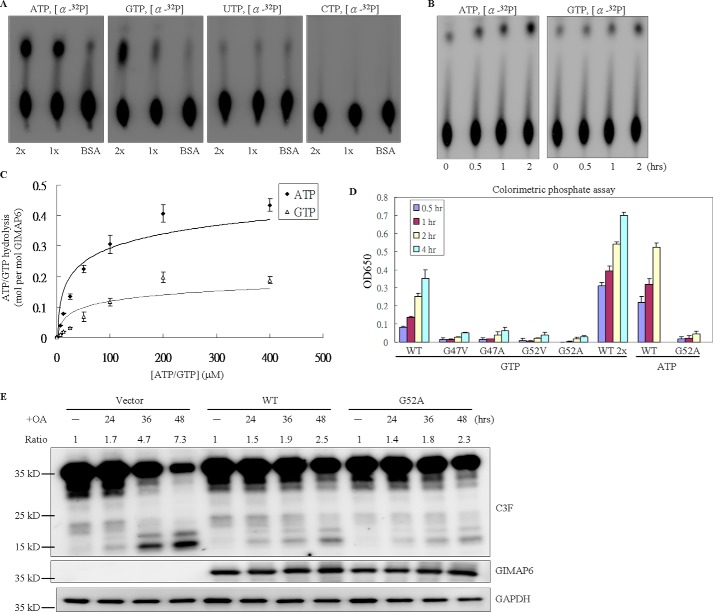
Figure 4.
Hydrolysis activity of GIMAP6 for ATP and GTP is not associated with the antiapoptotic function. A, the intrinsic hydrolysis activity of the indicated protein was measured in the presence of 2 mm MgCl2 for 2 h at 37 °C by TLC analysis. Specifically, 33 nm of α-32P-labeled ATP, GTP, UTP, and CTP were used separately as substrates, and then hydrolysis activity was measured by TLC. 1x and 2x indicate the relative amounts of GIMAP6 and BSA, used as the control. B, ATP and GTP hydrolysis activity time course. The reaction was incubated at 37 °C. At the indicated time points, a sample of the reaction product (0.5 μl) was removed and stopped by adding 250 μm EDTA, followed by incubation at 95 °C on a heating block for 5 min. C, rate of ATP/GTP hydrolysis by GIMAP6 in relation to the ATP/GTP concentration. To obtain the Kcat data, a saturation curve was obtained by using increasing substrate concentrations under hydrolysis conditions when there was a constant concentration of GIMAP6. Rates of ATP/GTP hydrolysis by GIMAP6 were determined by Lineweaver-Burk plot. D, the GTP/ATP hydrolysis activity of various GIMAP6 mutants (G47V, G47A, G52V, and G52A) was greatly reduced compared with that of the wild-type GIMAP6 protein (WT) using a colorimetric phosphate detection assay. The data represent the average of two independent experiments with triplicate measurements (mean ± S.D.). E, the anti-apoptotic effect of the G52A mutant compared with that of the wild-type protein. A transient transfection assay was conducted using Huh-7 cells and pcDNA3.1(+) (Vector). Apoptosis, which was induced by OA treatment, was measured by immunoblotting against the C3F antibody (which recognizes both the full-length and cleaved form of caspase-3; GeneTex, GTX110543). WT and G52A denote cells transiently expressing wild-type GIMAP6 and G52A mutant protein, respectively. Note that, 36 and 48 h after OA treatment, the expression level of the active-form caspase-3 was reduced to the same level as that of the wild-type GIMAP6 and the G52A mutant. The caspase-3 cleavage was calculated as cleaved/(cleaved + full-length) caspase-3, and the relative signal intensity (Ratio) in the untreated lane (−) for each group was taken as 1.