Figure 1.
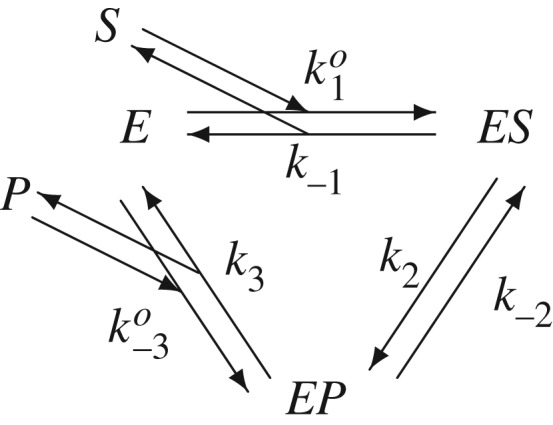
Kinetic scheme for a reversible enzymatic reaction with substrate S and product P. k−1, k2, k−2 and k3 are first-order rate constants; and ko1 and ko−3 are second-order rate constants. The system of biochemical reactions is in equilibrium if and only if the concentrations of S and P, [S] and [P], satisfy k1k2k3 = k−3k−2k−1, in which pseudo-first-order rate constants k1 = ko1[S] and k−3 = ko−3[P].
