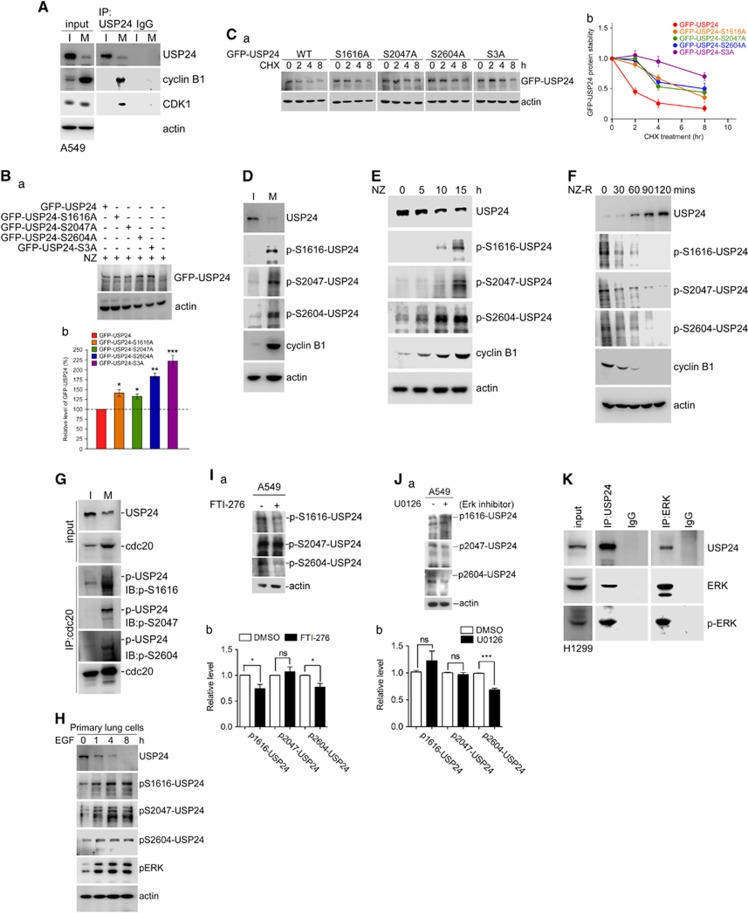
Figure 7.
Regulation of USP24 by phosphorylation increases its degradation. (A) Samples were collected from A549 cells that remained in interphase (I) and mitosis (M) for the immunoprecipitation assay with an anti-USP24 antibody. The immunoprecipitated (IP) samples were analyzed by western blotting with antibodies against USP24, CDK1, cyclin B1 and actin. (B) Various mutations of USP24 phosphorylation residues, including a triple mutant (S3A), were constructed and expressed in cells. Samples were analyzed by western blotting with an anti-GFP antibody (a). The GFP-USP24 level was quantified after three independent experiments (b). (C) The proteins stabilities of USP24-wt (WT) and its mutants (S1616A, S2047A, S2604A and S3A) were investigated after cycloheximide (CHX) treatment. Samples were analyzed by western blotting with an anti-GFP antibody (a). Proteins levels were quantified after three independent experiments (b). (D) Normal or mitotic A549 cells were collected for an immunoblotting assay with antibodies against USP24, phospho-USP24 (S1616), phospho-USP24 (S2047), phospho-USP24 (S2604), cyclin B1 and actin. (E, F) Samples were collected under nocodazole treatment (NZ) (E) or nocodazole release (NZ-R) (F). Samples were analyzed with antibodies against USP24 phosphorylation at indicated residues, cyclin B1 and actin. (G) A549 cells treated with nocodazole were collected for an immunoprecipitation assay with anti-cdc20. The immunoprecipitated samples were used for western blotting with antibodies against the indicated proteins. (H) Samples collected from EGF-treated mice lung primary cells were analyzed by western blotting with antibodies against indicated proteins. (I) Samples were collected from A549 cells treated with FTI-276, and then analyzed by western blotting with antibodies against USP24 phosphorylation at the indicated residues (a). The relative levels were quantified after three independent experiments (b). (J) Samples were collected from A549 cells treated with Erk inhibitor, and then analyzed by western blotting with antibodies against USP24 phosphorylation at the indicated residues (a). The relative levels were quantified after three independent experiments (b). (K) Samples were collected from H1299 cells for immunoprecipitation with anti-USP24 and anti-ERK antibodies, and then immunoprecipitated samples were used to study the USP24, Erk and p-Erk levels by western blotting with anti-USP24, anti-Erk and anti-p-Erk antibodies.