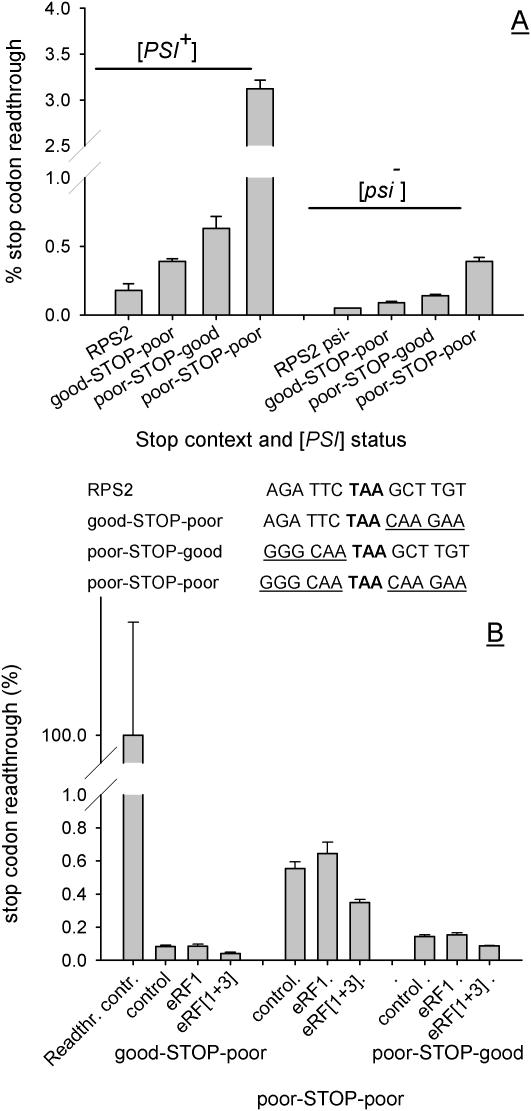
Figure 3.
Stop codon readthrough directed by either poor 5′ or 3′ context is identically responsive to release factor levels. Sequences of 6 nt representing poor 5′ or 3′ stop codon contexts were used singly or in combination to replace the stop codon context elements of the RPS2 gene [hybrid sequences listed in (A)], and cloned into a dicistronic stop codon readthrough vector system (Materials and Methods). (A) Plasmids pAC98-RPS2, pAC98-GP, pAC98-PP and pAC98-PG were transformed into the [PSI+] and [psi−] derivatives of yeast strain 76D694, and levels of stop codon readthrough determined. (B) Levels of stop codon readthrough were measured in the same strains additionally transformed with either the pRS426 and YEp24 vectors (labelled control), pRS426 with the multicopy eRF1 vector pUKC802 (labelled eRF1), or pJR16 and pUKC802 which together direct over-expression of both release factors (labelled eRF[1+3]). For (A and B), the mean of three readthrough determinations is shown (bars represent the SD, n = 3).