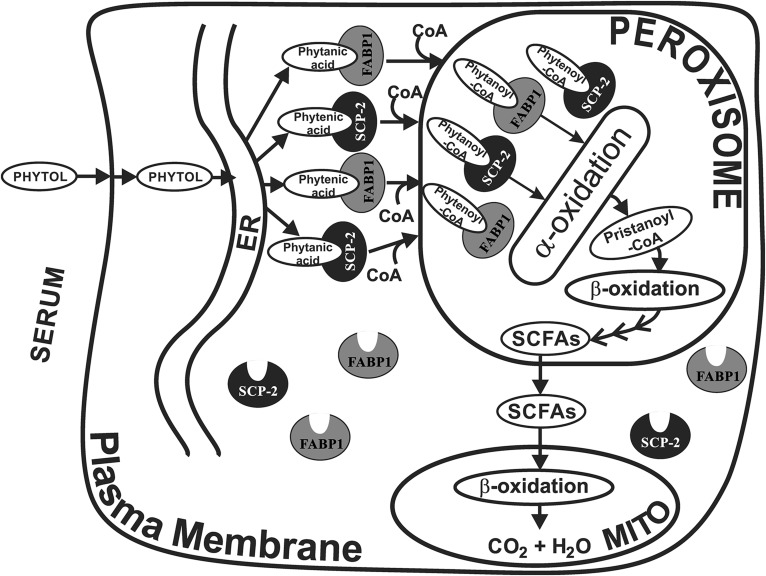
Fig. 7.
Schematic of proposed FABP1 and SCP-2/SCP-x interactions with the phytol metabolic pathway. FABP1 and SCP-2, both present at high level in hepatic cytoplasm, bind phytol metabolites, but not phytol. In this hypothetical scheme, FABP1 and SCP-2 bind phytanic acid and phytenic acid (formed from phytol in the endoplasmic reticulum) and transport them to the peroxisome. At the peroxisomal membrane the phytanic and phytenic acid are converted to their respective CoA thioesters, internalized and desorbed into the peroxisomal matrix wherein they are bound (20, 21) by peroxisomal localized SCP-2 (9–11) and potentially FABP1 (34). Within the peroxisomal matrix, SCP-2 directly interacts with oxidative enzymes and stimulates phytanoyl-CoA 2-hydroxylase, the essential enzyme mediating the first step in peroxisomal α-oxidation of branched-chain fatty acids (8, 19). SCP-x, an exclusively peroxisomal protein, is the only known branched-chain 3-ketoacyl CoA thiolase (10, 22–24). Successive cycles of α- and β-oxidation shortens the branched-chain fatty acids to yield short-chain (<C12) fatty acids (SCFAs), such as formyl-CoA, acetyl-CoA, propionyl-CoA, and 4,8-dimethyl nonanoyl-CoA. Neither FABP1 (38, 86–88) nor SCP-2 (20, 40) binds short-chain (<C12) fatty acids or their CoA thioesters.