Abstract
Objective:
This research was conducted to explore mechanisms behind the potency of quercetin in inhibiting colon cancer induced in an experimental model.
Materials and Methods:
Forty adult male rats of Wistar strain were distributed into 4 groups; a negative control group, a colon cancer bearing group, a quercetin-treated group and a 5-fluorouracil (5-FU)-treated group. Serum TAG72 and GAL3 levels were quantified by ELISA. Colonic Wnt5a and Axin-1 gene expression was estimated by PCR. In addition, colonic tissues were subjected to immunohistochemical examination of Bax expression and histological investigation of histopathological alterations.
Results:
Quercetin elicited significant reduction in serum TAG72 and GAL3 levels, in addition to significant suppression of colonic Wnt5a gene expression and amplification of colonic Axin-1 gene expression. Also, it caused moderate positive reaction for Bax in mucosal epithelium.
Conclusion:
The present research provides experimental evidence about the activity of quercetin in the colon cancer of rats. Inhibitory effects on cancer development might be ascribable to regulatory action on Wnt signaling and induction of apoptosis.
Keywords: Colon cancer, quercetin, 5-fluorouracil, Wnt signaling, rats
Introduction
Colon cancer represents one of the most causes of malignancy related morbidity and mortality around the world. In this process genetic and epigenetic alterations were included to impart tumor cells with a selective character to amplify the clones (Gellad and Provenzale, 2010).
The interaction between genetic and environmental factors as well as consumption of high-fat diet are considered as causative factors of colon cancer (Endo et al., 2009). Also, life way propensities like physical inactivity, stress, consumption of alcohol and ingestion of red meat increase the risk of colon cancer significantly. Late record indicated that out of 141,210 cases, about 49,380 cases had died, making colon cancer the third most common reason of cancer deaths globally (Balaji et al., 2014).
The onset of colon cancer is related to the presence of aberrant crypt foci (ACF) that progresses into polyps then adenomas and adenocarcinomas (Cappell, 2007). This carcinomas is often associated with inflammation (Steinberg, 2010). This cascade of events is thought to be a result of numerous genetic changes in the epithelium of colon along the Wnt signaling axis. The de-regulation of Wnt signaling via hereditary, environmental or other stimuli promotes an oncogenic gene expression program that implicates to colon carcinogenesis (Barker and Clevers, 2006).
Chemotherapy using 5-FU is usually applied for treatment of colon cancer patients. Despite the improvement in response rate due to the combinations of 5-FU with irinotecan and oxaliplatin, these chemotherapeutic agents showed no putative effect on disseminated colon cancer because of drug resistance. This opened up a line of sought about the specific needs for finding new therapeutic candidates that have the ability to overcome drug resistance in order to manage this type of malignancy (Raftery and Goldberg, 2010). In a sample of patients, the application of cytoreductive surgery in association with hyperthermic intraperitoneal chemoperfusion has shown promising results (Rampone et al., 2010). Otherwise, accumulating evidences indicate that natural products can modify different molecular cascade of events implicated in the initiation and progression of cancer (Rajamanickam and Agarwal, 2008).
A phytochemical rich eating regimen utilization (fruits and vegetables) has been linked with a reduction of colon cancer risk (van Duijnhoven et al., 2009). Among foods, little fruits and berries have pulled in great attention, and the correlation between their bioactive constituents and tumor counteractive action has gotten sharp experimental interest (Seeram, 2008; Stoner, 2009).
Quercetin (2-(3,4 dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one) which is one of the most representative compound of the flavonol subclass of flavonoids has beneficial potency in the protection from diseases, including cancer (Bulzomi et al., 2012). This compound caused apoptosis and cell cycle arrest in various cell sorts involving prostate, glioblastoma, breast, and colon cancer cell lines (Kuo et al., 2004; Vijayababu et al., 2006; Kim et al., 2008; Mense et al., 2008; Galluzzo et al., 2009). Multiple mechanisms have been proposed to elucidate these impacts of quercetin, including strand breakage of DNA, restraint of enzymes and kinases associated with survival signal transduction axis [e.g., PI3 kinase, protein kinase C, extracellular signal-regulated kinase (ERK)], and anti- or pro-oxidant activity (Kim et al., 2004; Vijayababu et al., 2006; Stevenson and Hurst, 2007). Accordingly, the current study sought to inspect the intimate mechanisms behind the efficacy of quercetin in repressing colon cancer in the experimental animals.
Materials and Methods
Extraction, preparation and isolation of quercetin compound from Punica granatum peel
Punica granatum peels were authenticated and extracted according to previously published work (Ahmed et al., 2015). Then, the extract was filtered, evaporated under reduced pressure, lyophilized and loaded on a polyamide 6S column chromatography (80 x 3 cm). The column was eluted with H2O, and then H2O-CH3OH mixtures of decreasing polarity and 10 fractions (1 L, each) were collected. The obtained phenolic fraction was subjected to column chromatography on cellulose and n-butanol (n-BuOH) saturated with H2O as an eluent to give sub-fraction which further fractionated on a Sephadex LH-20 to yield quercetin compound. The chemical structure of quercetin was identified using nuclear magnetic resonance (1H NMR) spectroscopy and carbon-13 nuclear magnetic resonance (13C NMR) spectroscopy.
Chemicals and Drugs
N-methylnitrosourea was supplied from Sigma-Aldrich Chemicals Co., (USA). While, 5-FU was purchased from EBEWE Pharma Ges.m.b.H. Nfg.KG, (Austria). All the other chemicals were of high purity grade in accordance with international standards.
Rats
Adult male rats of Wistar strain weighing 170-200 g were supplied from the Animal House Colony of the National Research Centre, Giza, Egypt and housed in polypropylene cages in an environmentally controlled clean air room with a temperature of 25±1°C, an alternating 12h light/12h dark cycle, a relative humidity of 60±5% and ad libitum access to tap water and a standard rodent chow consisted of 10% casein, 4% salt mixture, 1% vitamin mixture, 10% corn oil, 5% cellulose and completed to 100 g with corn starch (Wadi El Kabda Co., Cairo, Egypt). Rats were allowed to adapt to their environment for at least 2 weeks before the initiation of the experiment. The experimental protocols were carried out according to the guidelines for animal experiment, which was approved by the Ethical Committee of Medical Research of National Research Centre, Giza, Egypt.
Experimental setting
In this study, forty rats were randomly allocated into 4 groups, with 10 rats in each group. Group (1): set as healthy control group [negative control]. Group (2): set as colon cancer bearing group [colon cancer group] in which the rats were intrarectally injected with N-methylnitrosourea in a dose of 2 mg dissolved in 0.5 ml water/rat five times weekly for 6 weeks (Narisawa and Fukaura, 2003). Group (3): Quercetin-treated group [colon cancer + Quercetin] in which colon cancer bearing rats were treated orally with quercetin compound in a dose of 50 mg/kg b.wt (Zamin et al., 2014) daily for 8 weeks. Group (4): 5-FU-treated group [colon cancer + 5-FU] in which colon cancer bearing rats were intraperitoneally treated with 5-FU as reference drug in a dose of 12.5 mg/kg b. wt on days 1, 3 and 5 with the cycle being repeated every 4 weeks over the duration of the study period (8 weeks) (Asao et al., 1992).
After rat treatment was over, rats were fasted overnight and the blood samples were collected, under diethyl ether anesthesia, from the retro-orbital venous plexus in a dry clean centrifuge tubes and allowed to coagulate for 45 min at room temperature to obtain sera for biochemical analyses. Serum samples were separated by centrifugation at 1800 x g for 15 min at 4 °C using cooling centrifuge and maintained at -20 °C until analyzed. After collection of the blood samples, the animals were sacrificed by cervical dislocation and colon tissue of each rat was dissected and divided into two portions, the first portion was collected in liquid nitrogen and retained at -80° C for molecular genetics analysis and the second portion was placed in PBS-formalin solution (10%) for immunohistochemical examination and histological investigation.
Biochemical determinations
Serum tumor associated glycoprotein 72 (TAG72) and galectin-3 (GAL3) levels were estimated by enzyme-linked immunosorbent assay (ELISA) using the kits purchased from Glory Science Co., (USA), according to the manufacturer’s instructions provided with the kits.
Molecular study
Total RNA was extracted from the colon tissues of rats in each group using Trizol reagent (Bioshop Canada Inc., Canada). Isolated RNA was reverse transcribed into cDNA using RevertAid first strand cDNA synthesis kit (Fermentas, USA). The subsequent PCR was performed using 5 µg of cDNA in a final volume of 20 μl containing 10x PCR buffer, 10 mM dNTPs, 5 U/μl of Taq DNA polymerase (Fermentas) and 10 µM of each specific primers. Glyceraldhyde 3 phosphate dehydrogenase (GAPDH) was used as an internal control. The PCR cycling was performed using a gradient thermal cycler (BioRad, USA) as follow; (1) initial denaturation at 94ºC for 5 min, (2) Amplification with denaturation at 94ºC for 30 sec, annealing for 30 sec at (58ºC for Wnt5a and GAPDH, and 50ºC for Axin-1) for 35 cycles, and an extension step at 72ºC for 1 min, followed by (3) a final extension at 72ºC for 8 min. The PCR products were subsequently separated by 2% agarose gel electrophoresis followed by visualization using the gel documentation system (BioRad). The amplified product size was determined by comparison to DNA ladder (100 bp) (Fermentas). All gene expression levels were semi-quantified using Lab Image analysis (Lab Image2.7.0, Kapelan GmbH) software and were normalized against GAPDH gene expression. The primer pairs for Wnt5a and Axin-1 genes were selected according to the published sequences of Sadik and Shaker, (2013) and GAPDH gene (Paul and Kundu, 2013) were synthesized by Metabion, (Germany). The PCR primer sequences and their product size are listed in Table (1).
Table 1.
List of Primers Used in PCR.
| Gene primer sequences | Product size | |
|---|---|---|
| GAPDH | F: 5-`CAAGGTCATCCATGACAACTTTG-3` | 496 bp |
| R: 5`GTCCACCACCCTGTTGCTGTAG-3` | ||
| Wnt5a | F: 5’-CATCTGCCAGGTTGTATACTGTCC-3’ | 324 bp |
| R: 5’-TGATGCAAATAGGCAGCCGAGAGA-3’ | ||
| Axin-1 | F: 5’-TGCAGAGTCCCAAAATGAATG-3’ | 658 bp |
| R: 5’-GAGCCTGTCCTTGTGTAC-3’ |
Immunohistochemical examination of colonic Bax expression
Samples were taken from colon tissue of rats of the different groups and fixed in 10% PBS-formalin solution for 24 h. Washing was done in tap water then ascending grade of ethyl alcohol (30, 50, 70, 90% and absolute) was used for dehydration. Specimens were cleared in xylene and embedded in paraffin (melting point 58-60 ºC) for 24 h. Sections were cut into 4 µ thick by slidge microtome then fixed on positive slides in a 65 °C Oven for 1 h. Slides were placed in a coplin jar filled with 200 ml of triology working solution (Cell Marque, USA) which combines the three pretreatment steps: deparaffinization, rehydration and antigen unmasking. Then, the jar is securely positioned in the autoclave which was adjusted so that temperature reached 120 °C and maintained stable for 15 min after which pressure is released. Thereafter, the coplin jar is removed to allow slides to cool for 30 min. Sections were then washed and immersed in Tris-buffer saline (TBS) to adjust the pH and these were repeated between each step of the IHC procedure. Quenching endogenous peroxidase activity was performed by immersing slides in 3% hydrogen peroxide for 10 min. Broad spectrum LAB-SA detection system (Invitrogen, USA) was used to visualize any antigen-antibody reaction in the tissue. Background staining was blocked by putting 3 drops of 10% goat non immune serum blocker on each slide and incubating them in a humidity chamber for 10 min. Without washing, excess serum was drained, three drops of Bax mouse monoclonal antibody (Thermo Scientific, USA) were applied and slides were incubated in the humidity chamber overnight at 4 °C. Henceforward, biotinylated secondary antibody from ultravision detection system anti-polyvalent HRP/DAB (Thermo Scientific) was applied on each slide for 20 min followed by 20 min incubation with the streptavidin HRP enzyme conjugate (Thermo Scientific). Then, 3,3´-diaminobenzidine (DAB) chromogen (Thermo Scientific) was prepared and 3 drops were applied on each slide for 2 min. DAB was rinsed, after which counterstaining with Mayer hematoxylin and cover slipping were performed as the final steps before slides were examined under the light microscope (Bancroft and Gamble, 2008). Image analysis was performed using the image J, 1.41a NIH, (USA) analyzer.
Histological investigation of colon tissue sections
After fixation of colon tissues in PBS-formalin solution (10%) for 24 h, the tissues were washed in running tap water and then ascending grade of ethyl alcohol (30, 50, 70, 90% and absolute) was used for dehydration. Specimens were cleared in xylene and embedded in paraffin (melting point 58-60 ºC) for 24 h. Paraffin wax tissue blocks were prepared for sectioning at 4 µ by slidge microtome. The obtained tissue sections were collected on glass slides, deparffinized and stained by hematoxylin and eosin (H and E) stain (Banchroft et al., 1996) for histopathological examination through the electric light microscope.
Statistical analysis
The obtained results are represented as the means ± standard errors. Data were analyzed by one way analysis of variance (ANOVA) using the Statistical Package for the Social Sciences (SPSS) program, version 14 followed by least significant difference (LSD) to compare significance between groups. The level of significance was set at P< 0.05.
Results
Identification of the isolated compound
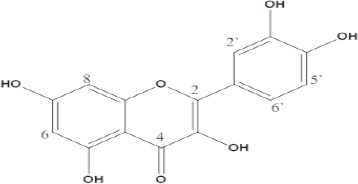
The isolated compound (quercetin) was amorphous yellow powder, a dull yellow color under UV light gave bright yellow with either ammonia vapor or aluminum chloride (AlCl3). UV spectral data of the isolated compound revealed two major absorption bands in methanol band I at 370 nm and band II at 256 nm, which is a flavonol with a free OH at position 3 and 5 (Harborne, 1984). The addition of sodium methoxide (NaOMe) makes a bathochromic shift (+∆58) in band I with an decrease in intensity, which decomposed after 5 min proved the presence of a free OH groups at position 3, 3’ and 4’ (Mabry et al., 1970). The addition of sodium acetate (NaOAc) makes a bathochomic shift (+∆20) in band II compared with the same band in methanol and the presence of a shoulder at 331 nm in NaOMe suggested the presence of a free OH group at position 7. The bathochromic shift in band I (+∆72) on addition of AlCl3 indicated the presence of free OH group at position 5, while the hypthochromic shift which occurred on the addition of HCl in band I suggested the presence of a free orthodihydroxy groups in the B-ring (Marbry et al., 1970; Markham, 1982).
The 1H NMR showed the aromatic proton of the B- ring as doublet at δ 7.69, J = 2.1 Hz of H-2’ due to meta coupling of H-6’ and doublet of doublet at δ 7.55, J = 2.1 Hz and 8.4 Hz of H-6’ due to meta coupling with H-2’ and ortho- coupling with H-5’ respectively, a doublet at (δ 6.9, J = 8.4 Hz) for H-5’ due to an ortho coupling with H-6’ was observed, two aromatic proton of the A-ring showed as two doublet at δ 6.20 and δ 6.42 with J = 1.8 Hz of each proton due to meta coupling of H-6 and H-8 respectively. Meanwhile, 13C NMR displayed 15 carbons as δ (ppm) was 147.50 (C-2), 136.44 (C-3), 176.55 (C-4), 161.43 (C- 5), 98.88 (C-6), 164.59 (C-7), 94.05 (C-8), 156.83 (C-9), 103.71 (C-10), 122.66 (C-1’), 116.31 (C-2’), 145.76 (C-3’), 148.40 (C-4’). These findings are found to be in accordance with the proposed structure of quercetin (Zheng et al., 2008).
Biochemical markers
Table (2) showed the influence of treatment with quercetin compound and 5-FU on serum TAG72 and GAL3 levels in colon cancer rat model. The colon cancer bearing rats showed significant rise (P< 0.05) in serum TAG72 and GAL3 values versus the negative controls. Contrariwise, treatment of colon cancer bearing rats with quercetin compound or 5-FU caused significant drop (P< 0.05) in serum TAG72 and GAL3 values in respect with the untreated colon cancer bearing rats.
Table 2.
Influence of Treatment with Quercetin Compound and 5-FU on Serum TAG72 and GAL3 Levels in Colon Cancer Rat Model
| Items | TAG72 (pg/mL) | GAL3 (ng/mL) |
|---|---|---|
| Groups | ||
| Negative control | 1,533.2 ± 30.9 | 3.2 ± 0.051 |
| Colon cancer | 2,325 ± 191.5a | 6.1 ± 0.15a |
| Colon cancer + Quercetin | 1,642.9 ± 31.9b | 4.9 ± 0.23b |
| Colon cancer + 5-FU | 1,604 ± 30.5b | 4.8 ± 0.22b |
Significant difference at P< 0.05 compared with negative control group;
Significant difference at P< 0.05 compared with colon cancer bearing group
Molecular genetics markers
Figures (1a) and (1b) as well as Table (3) showed the influence of treatment with quercetin compound and 5-FU on colonic Wnt5a and Axin-1 genes expression in colon cancer rat model. The colon cancer bearing rats displayed significant amplification (P< 0.05) in the expression of colonic Wnt5a gene paralleled by significant suppression (P< 0.05) in the expression of colonic Axin-1 gene relative to the negative controls. While, treatment of colon cancer bearing rats with quercetin compound or 5-FU led to significant suppression (P< 0.05) in the expression of colonic Wnt5a gene in concomitant with significant amplification (P< 0.05) in the expression of colonic Axin-1 gene relative to the untreated colon cancer bearing rats.
Figure 1.
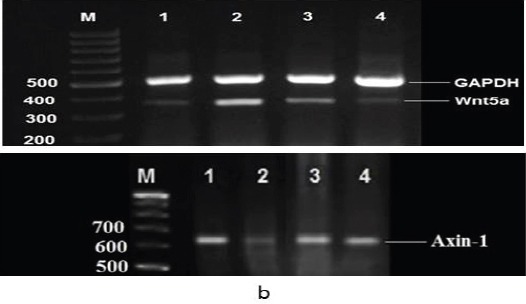
Agarose Gel Electrophoresis Showing (a): Wnt5a and (b): Axin-1 mRNA expression in the colon tissue by RT-PCR analysis. GAPDH expression with 496 bp, Wnt5a expression with 324 bp and Axin-1 expression with 658 bp. Lane (1): represents negative control group and lane (2): represents colon cancer bearing group. Lane (3): represents colon cancer + quercetin group, whereas lane (4) represents colon cancer + 5-FU group. Lane M: represents DNA ladder (100 bp).
Table 3.
Influence of Treatment with Quercetin Compound and 5-FU on Colonic Wnt5a and Axin-1 Genes Expression in Colon Cancer Rat Model
| Items | Wnt5a Expression | Axin-1 Expression |
|---|---|---|
| Groups | ||
| Negative control | 0.25 ± 0.01 | 0.92 ± 0.018 |
| Colon cancer | 0.69 ± 0.038a | 0.78 ± 0.017a |
| Colon cancer + Quercetin | 0.29 ± 0.015b | 0.81 ± 0.02b |
| Colon cancer + 5-FU | 0.29 ± 0.016b | 0.81 ± 0.02b |
Significant difference at P< 0.05 compared with negative control group;
Significant difference at P< 0.05 compared with colon cancer bearing group
Immunohistochemical marker
Photomicrograph for immunohistochemical staining of colon tissue section of negative control rat using antibody against Bax showed moderate positive reaction in mucosal epithelium (Figure 2a). While, that of colon cancer bearing rat showed mild positive reaction in mucosal epithelium (Figure 2b). Photomicrographs for immunohistochemical staining of colon tissue sections of colon cancer bearing rats treated with quercetin compound or 5-FU showed moderate positive reaction in mucosal epithelium (Figure 2c and d respectively).
Figure 2.
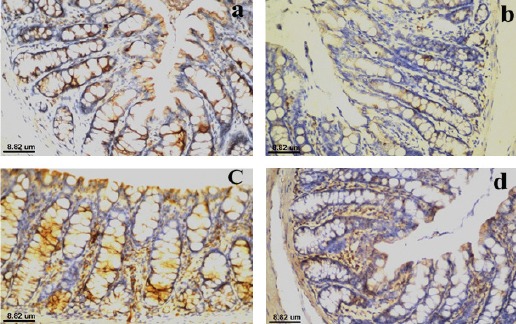
Photomicrograph for Immunohistochemical Staining of Colon Tissue Section of (a): negative control rat using antibody against Bax showed moderate positive reaction in mucosal epithelium, (b): colon cancer bearing rat using antibody against Bax showed mild positive reaction in mucosal epithelium, (c): colon cancer bearing rat treated with quercetin compound using antibody against Bax showed moderate positive reaction in mucosal epithelium and (d): colon cancer bearing rat treated with 5-FU using antibody against Bax showed moderate positive reaction in mucosal epithelium.
Histological investigations
Photomicrograph of colon tissue section of negative control rat showed normal histological structure of mucosa as observed in Figure (3a). While, that of colon cancer bearing rat showed absence of the crypts as well as goblet cells of the mucosal epithelium with oval shape deep basophilic hyperchromatic, karyomegalic polar nuclei, irregular arrangement (dysplasia) and in focal multiple numbers (hyperplasia) (Figure 3b). However, photomicrograph of colon tissue section of colon cancer bearing rat treated with quercetin compound showed intact glandular mucosal structure as well as inflammatory cells infiltration within lamina propria (Figure 3c). Finally, photomicrograph of colon tissue section of colon cancer bearing rat treated with 5-FU showed normal histological structure of mucosa as shown in Figure (3d).
Figure 3.
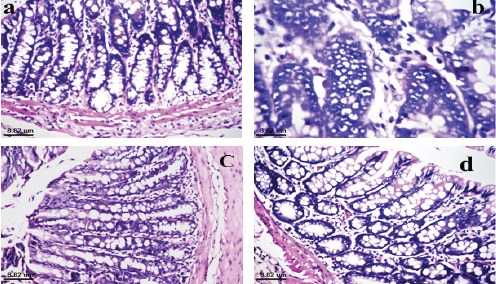
Photomicrograph of Colon Tissue Section of (a): negative control rat showed normal histological structure of mucosa, (b): colon cancer bearing rat showed absence of the crypts and goblet cells of mucosa with dysplastic, hyperchromatic irregular arrangement of nuclei with hyperplasia, (c): colon cancer bearing rat treated with quercetin compound showed normal histological structure of glandular epithelium with few inflammatory cells infiltration in the lamina propria and (d): colon cancer bearing rat treated with 5-FU showed normal histological structure of mucosa.
Discussion
Tabulated results of this work indicated significant amplification in the serum level of TAG72 and GAL3 in colon cancer bearing rats. Guadagni et al., (1996) reported that TAG72 is expressed in more than 80% of colorectal carcinomas and its serum levels correlate with the stage of disease, suggesting its utility in differentiating between early-stage against late-stage colon carcinoma. Furthermore, Song et al., (2014) mentioned that GAL3 is associated with the development of several cancers including colorectal cancer. GAL3 is an oncogenic protein which directs cell development, adhesion (Hughes, 2001), proliferation and apoptosis, as well as cell-cell interaction and angiogenesis (Zou et al., 2005; Nakahara and Raz, 2007). Supporting evidences have documented that GAL3 is expressed significantly in metastatic cancer cells, and elevated expression of GAL3 can be recorded in primary and metastatic lesions (Iurisci et al., 2000), even in blood (Greco et al., 2004), indicating a high relation with cancer growth and metastasis (Zhang et al., 2006). Cheong et al., (2010) cited that GAL3 is highly expressed in human cancers. In earlier studies, cellular expression of GAL3 was elevated in many cancer types, including gastric cancer (Puglisi et al., 2004; Ahmed et al., 2007). Moreover, Endo et al., (2005) reported that GAL3 expression can be utilized as a prognostic and diagnostic tool of colon cancer.
In view of the current data, treatment of colon cancer bearing rats with quercetin compound led to significant decline in serum TAG72 and GAL3 levels. Agullo et al. (1994) and Scambia et al., (1994) mentioned that quercetin applies a particular cytotoxic impact on colon carcinoma (HT29 and CaCo2 cells). Furthermore, quercetin instigates apoptosis in human leukemia (HL60 cells) following growth suppression. These effects could be ascribed to amplified expression of wild type p53 (Plaumann et al., 1996), and inhibition of Ki ras levels (Csokay et al., 1997), or p21 amplification (Narayanan et al., 1999). Also, Uddin and Choudhry (1995) and Narayanan et al., (1999) found that quercetin inhibits the synthesis of DNA in HL60 cells. Many mechanisms behind the anti-carcinogenic impact of flavonoids have been proposed. For example, antioxidant capacity, modulation of the activity of enzymes correlated with carcinogen stimulation, regulation of gene expression, and P-glycoprotein potentiation (Duthie et al., 2000). In addition, the antitumor potential of flavonols was initially credited to their donation of electron, which stems from phenolic OH groups. Meanwhile, a rising perspective is that flavonoids, including quercetin, may likewise exhibit modulatory actions in cells via protein kinase and lipid kinase signaling axis (Kiss and Dubois, 2011).
Characterizing protein molecules that manage Wnt axis have uncovered critical bits of knowledge into Wnt-dependent processes (Chien and Moon, 2007). It has been reported that stabilization of β-catenin represents the vital event in Wnt signaling (Clements et al., 2003). Without Wnt ligands, β-catenin levels are suppressed by the adenomatous polyposis coli (APC)-Axin-glycogen synthase kinase 3 beta (GSK3β) protein complex (Logan and Nusse, 2004). Initiation of the canonical Wnt signaling axis entails Wnt ligands binding to frizzled (FRZ) receptors (Pinson et al., 2000). This results in phosphorylation of disheveled proteins with subsequent inactivation of GSK3β (Lee et al., 1999). Suppressed GSK3β cannot perceive β-catenin to phosphorylate serine and threonine residues, thus protecting β-catenin from proteolytic decay (Orford et al., 1997).
In light on the current data, Wnt5a gene was amplified significantly whereas Axin-1 gene was suppressed in colonic tissues of colon cancer bearing rats. These findings match those of Sadik and Shaker (2013). Wnt5a is a secreted proto-oncogenic glycoproteins whose amplification and binding to FRZ cell-surface receptors has been demonstrated in human tumor cells (Katoh et al., 2003). Also, dysfunction and mutations in Axin and earn of functional mutations in β-catenin have been recorded in various cancers (Reya and Clevers, 2005; Klaus and Birchmeier, 2008). Indeed, numerous cancers show elevated β-catenin levels with an evidently genetically fit Wnt axis (Bafico et al., 2004). Abnormality of Wnt/β-catenin signaling axis can develop about 60–80 % of colon cancers. Accumulation of β-catenin in the tumor cells is the indicator of Wnt/β-catenin signaling axis dysregulation (Clevers, 2006). Axin-1 is a tumor suppressor in the Wnt axis that functions to down-regulate signaling. Axin is negative regulator of the Wnt signaling axis, which stimulates the phosphorylation and decay of β-catenin (Kikuchi, 1999). It functions as a scaffold to bind β-catenin, GSK3β, and APC, and permits the phosphorylation of β-catenin.
Our study showed that treatment of colon cancer bearing rats with quercetin compound inhibited the overexpression of Wnt5a and up-regulated the expression of Axin-1. Park et al. (2005) stated that quercetin inhibits the transcriptional activity of β-catenin/Tcf in SW480 and HEK293 cells transiently transfected with constitutively active mutant β-catenin gene. Also, these investigators reported that binding of the Tcf complexes to its specific DNA-binding sites is significantly inhibited by quercetin. Over and above, Lu et al. (2015) proposed that quercetin may potentially amplify phosphorylation of β-catenin by elevating Axin levels and thus regulate the Wnt/β-catenin signaling axis.
The reduction in Bax expression in the colon tissue of colon cancer bearing rats as shown in the immunohistochemical finding in the current study could be attributed to that Bax is considered to be one of target proteins implicating in the organization of proliferation and apoptosis, which is found to be disorganized in cancer cell cycles, and which has been shown to influence survival of colorectal carcinomas (Chen et al., 2007). Chen et al. (2007) speculated that cyclin D1, B-cell lymphoma 2 (Bcl-2) and Bax deviations may be manifested in the onset of colon cancer and the suppression of Bax expression could motivate the differentiation of cancer tissue into advanced stage.
Immunohistochemical examination of Bax expression in the colonic tissue of colon cancer bearing rats treated with quercetin revealed moderate positive reaction in mucosal epithelium. In consistent, Nguyen et al. (2004) found deviation in the balance between the anti-apoptotic and pro-apoptotic molecules upon treatment with quercetin. Probably, the reduction in Bcl-2 as a consequence of treatment with quercetin permits less Bcl-2-Bax complex. As a result, Bcl-2-associated death promoter (Bad) binds Bcl-2, and its pro-apoptotic action is significantly elevated. The rise of non-phosphorylated Bad due to quercetin further aids to segregate more Bcl-2 and B-cell lymphoma-extra large (Bcl-xL). The final result is more free Bax, which is transferred to the membrane of mitochondria and promotes mitochondrial permeability transition pore to be opened, a key event in the decrease of cell viability (Chao and Korsmeyer, 1998).
The photomicrograph of colon tissue section of untreated colon cancer bearing rats showed absence of the crypts as well as goblet cells of the mucosa with dysplastic, hyperchromatic irregular arrangement of nuclei with hyperplasia. These results fit similar findings reported by Narisawa and Fukaura (2003) and Ousingsawat et al. (2007). On the opposite hand, the photomicrograph of colon tissue section of colon cancer bearing rats treated with quercetin compound revealed normal histological structure of glandular epithelium with few inflammatory cells infiltration in the lamina propria. This preferable effect could be allied to the antioxidant, chemopreventive, anti-inflammatory and anti-proliferative properties of quercetin (Ramos, 2008).
Our data demonstrated that treatment of colon cancer bearing rats with 5-FU resulted in significant depletion in serum TAG72 and GAL3 levels. Also, 5-FU led to significant suppression in the expression of colonic Wnt5a gene associated with significant amplification in the expression of colonic Axin-1 gene. Furthermore, immunohistochemical examination of Bax expression in the colonic tissue of colon cancer bearing rats treated with 5-FU revealed moderate positive reaction in mucosal epithelium. Finally, the photomicrograph of colon tissue section of colon cancer bearing rats treated with 5-FU showed normal histological structure of mucosa. These effects could be explained by: 1) the anti-proliferative capacity of 5-FU (Noda et al., 2009), 2) its ability to regulate cell cycle progression by amplifying S-phase fraction (Eguchi et al., 2000), 3) its affinity to induce apoptosis by down-regulating Bcl-xl and Fas/FasL axis (Kondo et al., 2005; Damdinsuren et al., 2007; Nakamura et al., 2007; Nagano et al., 2007, He et al., 2011), 4) its capacity to modulate the immune response by inducing the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)/TRAIL-receptor axis (Yamamoto et al., 2004) and 5) its activity against tumor angiogenesis (Wada et al, 2007).
The current setup conferred experimental evidences that quercetin compound possessed a promising anticancer potential against colon cancer induced in rats. The underlying mechanisms could be related to its ability to exert preferential cytotoxic effect, regulate the altered Wnt signaling, motivate apoptosis and recover the structural organization of glandular epithelium of colon. These findings could pave the way for development of pharmaceutical formulations from quercetin to be tested on clinical trial levels for treatment of colon cancer.
References
- Agullo G, Gamet L, Besson C, Demigne C, Remesy C. Quercetin exerts a preferential cytotoxic effect on active dividing colon carcinoma HT29 and CaCO2 cells. Cancer Lett. 1994;87:55–63. doi: 10.1016/0304-3835(94)90409-x. [DOI] [PubMed] [Google Scholar]
- Ahmed H, Banerjee PP, Vasta GR. Differential expression of galectins in normal, benign and malignant prostate epithelial cells: silencing of galectin-3 expression in prostate cancer by its promoter methylation. Biochem Biophys Res Commun. 2007;358:241–6. doi: 10.1016/j.bbrc.2007.04.114. [DOI] [PubMed] [Google Scholar]
- Ahmed HH, Shousha WGh, El-Mezayen HA, El-Toumy SA, Ramadan AR. Updates on the biochemical and molecular mechanisms of N-nitrosodiethylamine-induced hepatocellular carcinoma: promising therapeutic role of Punica granatum peel extract. Int J Pharm Sci Rev Res. 2015;32:121–44. [Google Scholar]
- Asao T, Takayuki A, Shibata HR, Batist G, Brodt P. Eradication of hepatic metastases of carcinoma H-59 combination chemoimmunotherapy with liposomal muramyl tripeptide, 5-fluorouracil, and leucovorin. Cancer Res. 1992;52:6254–7. [PubMed] [Google Scholar]
- Bafico A, Liu G, Goldin L, Harris V, Aaronson SA. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell. 2004;6:497–506. doi: 10.1016/j.ccr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Balaji C, Muthukumaran J, Nalini N. Chemopreventive effect of sinapic acid on 1,2-dimethylhydrazineinduced experimental rat colon carcinogenesis. Hum Exp Toxicol. 2014;33:1253–68. doi: 10.1177/0960327114522501. [DOI] [PubMed] [Google Scholar]
- Banchroft JD, Stevens A, Turner DR. Theory and practice of histological techniques. 4th Ed. Philadelphia, USA: Churchill Livingstone; 1996. pp. 25–90. [Google Scholar]
- Bancroft JD, Gamble M. Theory and practice of histological techniques. 6th Ed. Philadelphia, USA: Churchill Livingstone; 2008. pp. 433–69. [Google Scholar]
- Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- Bulzomi P, Galluzzo P, Bolli A, et al. The pro-apoptotic effect of quercetin in cancer cell lines requires ERβ-dependent signals. J Cell Physiol. 2012;227:1891–8. doi: 10.1002/jcp.22917. [DOI] [PubMed] [Google Scholar]
- Cappell MS. From colonic polyps to colon cancer: pathophysiology, clinical presentation, screening and colonoscopic therapy. Minerva Gastroenterol Dietol. 2007;53:351–73. [PubMed] [Google Scholar]
- Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- Chen W, Lin M, Zhang B, et al. Survey of molecular profiling during human colon cancer development and progression by immunohistochemical staining on tissue microarray. World J Gastroenterol. 2007;13:699–708. doi: 10.3748/wjg.v13.i5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong T, Shin J, Chun K. Silencing of galectin-3 changes the gene expression and augments the sensitivity of gastric cancer cells to chemotherapeutic agents. Cancer Sci. 2010;101:94–102. doi: 10.1111/j.1349-7006.2009.01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien AJ, Moon RT. WNTs and WNT receptors as therapeutic tools and targets in human disease processes. Front Biosci. 2007;12:448–57. doi: 10.2741/2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements WM, Lowy AM, Groden J. Adenomatous polyposis coli/beta-catenin interaction and downstream targets: altered gene expression in gastrointestinal tumors. Clin Colorectal Cancer. 2003;3:113–20. doi: 10.3816/ccc.2003.n.018. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Csokay B, Pradja N, Weber G, Olah E. Molecular mechanisms in the antiproliferative action of quercetin. Life Sci. 1997;60:2157–63. doi: 10.1016/s0024-3205(97)00230-0. [DOI] [PubMed] [Google Scholar]
- Damdinsuren B, Nagano H, Wada H, et al. Interferon alpha receptors are important for antiproliferative effect of interferon-alpha against human hepatocellular carcinoma cells. Hepatol Res. 2007;37:77–83. doi: 10.1111/j.1872-034X.2007.00007.x. [DOI] [PubMed] [Google Scholar]
- Duthie GG, Duthie SJ, Kyle JA. Plant polyphenols in cancer and heart disease: implications as nutritional antioxidants. Nutr Res Rev. 2000;13:79–106. doi: 10.1079/095442200108729016. [DOI] [PubMed] [Google Scholar]
- Eguchi H, Nagano H, Yamamoto H, et al. Augmentation of antitumor activity of 5-fluorouracil by interferon alpha is associated with up-regulation of p27Kip1 in human hepatocellular carcinoma cells. Clin Cancer Res. 2000;6:2881–90. [PubMed] [Google Scholar]
- Endo H, Hosono K, Fujisawa T, et al. Involvement of JNK pathway in the promotion of the early stage of colorectal carcinogenesis under high-fat dietary conditions. Gut. 2009;58:1637–43. doi: 10.1136/gut.2009.183624. [DOI] [PubMed] [Google Scholar]
- Endo K, Kohnoe S, Tsujita E, et al. Galectin-3 expression is a potent prognostic marker in colorectal cancer. Anticancer Res. 2005;25:3117–21. [PubMed] [Google Scholar]
- Galluzzo P, Martini C, Bulzomi P, et al. Quercetin induced apoptotic cascade in cancer cells: Antioxidant versus estrogen receptor a dependent mechanisms. Mol Nutr Food Res. 2009;53:699–708. doi: 10.1002/mnfr.200800239. [DOI] [PubMed] [Google Scholar]
- Gellad ZF, Provenzale D. Colorectal cancer: national and international perspective on the burden of disease and public health impact. Gastroenterology. 2010;138:2177–90. doi: 10.1053/j.gastro.2010.01.056. [DOI] [PubMed] [Google Scholar]
- Greco C, Vona R, Cosimelli M, et al. Cell surface overexpression of galectin-3 and the presence of its ligand 90k in the blood plasma as determinants in colon neoplastic lesions. Glycobiology. 2004;14:783–92. doi: 10.1093/glycob/cwh092. [DOI] [PubMed] [Google Scholar]
- Guadagni F, Roselli M, Cosimelli M, et al. TAG-72 expression and its role in the biological evaluation of human colorectal cancer. Anticancer Res. 1996;16:2141–8. [PubMed] [Google Scholar]
- Harborne JB. Phytochemical methods. 2nd Ed. London: Chapman and Hall; 1984. pp. 1–36. [Google Scholar]
- He XT, Fan XM, Zha XL. Ghrelin inhibits 5-fluorouracil- induced apoptosis in colonic cancer cells. J Gastroenterol Hepatol. 2011;26:1169–73. doi: 10.1111/j.1440-1746.2011.06715.x. [DOI] [PubMed] [Google Scholar]
- Hughes RC. Galectins as modulators of cell adhesion. Biochimie. 2001;83:667–76. doi: 10.1016/s0300-9084(01)01289-5. [DOI] [PubMed] [Google Scholar]
- Iurisci I, Tinari N, Natoli C, et al. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res. 2000;6:1389–93. [PubMed] [Google Scholar]
- Katoh M. Expression and regulation of WNT1 in human cancer: up-regulation of WNT1 by beta-estradiol in MCF-7 cells. Int J Oncol. 2003;22:209–12. [PubMed] [Google Scholar]
- Kikuchi A. Roles of Axin in the Wnt signalling pathway. Cell Signal. 1999;11:777–88. doi: 10.1016/s0898-6568(99)00054-6. [DOI] [PubMed] [Google Scholar]
- Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci. 2004;96:229–45. doi: 10.1254/jphs.crj04003x. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim EH, Park SS, et al. Quercetin sensitizes human hepatoma cells to TRAIL-induced apoptosis via Sp1-mediated DR5 up-regulation and proteasome-mediated c-FLIPS down-regulation. J Cell Biochem. 2008;105:1386–98. doi: 10.1002/jcb.21958. [DOI] [PubMed] [Google Scholar]
- Kiss R, Dubois J. Quercetin inhibits a large panel of kinases implicated in cancer cell biology. Int J Oncol. 2011;38:833–42. doi: 10.3892/ijo.2010.890. [DOI] [PubMed] [Google Scholar]
- Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–98. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- Kondo M, Nagano H, Wada H, et al. Combination of IFN-alpha and 5-fluorouracil induces apoptosis through IFN-alpha/beta receptor in human hepatocellular carcinoma cells. Clin Cancer Res. 2005;11:1277–86. [PubMed] [Google Scholar]
- Kuo PC, Liu HF, Chao JI. Survivin and p53 modulate quercetin-induced cell growth inhibition and apoptosis in human lung carcinoma cells. J Biol Chem. 2004;279:55875–985. doi: 10.1074/jbc.M407985200. [DOI] [PubMed] [Google Scholar]
- Lee JS, Ishimoto A, Yanagawa S. Characterization of mouse disheveled (Dvl) proteins in Wnt/Wingless signaling pathway. J Biol Chem. 1999;274:21464–70. doi: 10.1074/jbc.274.30.21464. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lu H, Peng T, Hu X, et al. Quercetin potentiates the effect of γδT cells via modulating the expressions of Granzyme B, perforin and IFN-γand also regulates the Wnt/β-catenin signalling pathway in human colon cancer cells. Bangladesh J Pharmacol. 2015;10:251–9. [Google Scholar]
- Mabry TJ, Markham KR, Thomas MB. The Systematic identification of flavonoids. Verlag and New York: Springer; 1970. pp. 35–109. [Google Scholar]
- Markham KR. Techniques of flavonoid identification. London: Academic Press; 1982. pp. 1–113. [Google Scholar]
- Mense SM, Hei TK, Ganju RK, Bhat HK. Phytoestrogens and breast cancer prevention: Possible mechanisms of action. Environ Health Perspect. 2008;116:426–33. doi: 10.1289/ehp.10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano H, Miyamoto A, Wada H, et al. Interferon-alpha and 5-fluorouracil combination therapy after palliative hepatic resection in patients with advanced hepatocellular carcinoma, portal venous tumor thrombus in the major trunk, and multiple nodules. Cancer. 2007;110:2493–501. doi: 10.1002/cncr.23033. [DOI] [PubMed] [Google Scholar]
- Nakahara S, Raz A. Regulation of cancer-related gene expression by galectin-3 and the molecular mechanism of its nuclear import pathway. Cancer Metastasis Rev. 2007;26:605–10. doi: 10.1007/s10555-007-9095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Nagano H, Sakon M, et al. Role of the Fas/FasL pathway in combination therapy with interferon-alpha and fluorouracil against hepatocellular carcinoma in vitro. J Hepatol. 2007;46:77–88. doi: 10.1016/j.jhep.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Narayanan BA, Geoffroy O, Willingham MC, Re GG, Nixon DW. p53/p21 WAF1/CIP1 expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells. Cancer Lett. 1999;136:215–21. doi: 10.1016/s0304-3835(98)00323-1. [DOI] [PubMed] [Google Scholar]
- Narisawa T, Fukaura Y. Prevention by intrarectal 5-aminosalicylic acid of N-methylnitrosourea-induced colon cancer in F344 rats. Dis Colon Rectum. 2003;46:900–3. doi: 10.1007/s10350-004-6681-3. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Tran E, Nguyen TH, et al. The role of activated MEK-ERK pathway in quercetin-induced growth inhibition and apoptosis in A549 lung cancer cells. Carcinogenesis. 2004;25:647–59. doi: 10.1093/carcin/bgh052. [DOI] [PubMed] [Google Scholar]
- Noda T, Nagano H, Takemasa L, et al. Activation of Wnt/b-catenin signalling pathway induces chemoresistance to interferon-a/5-fluorouracil combination therapy for hepatocellular carcinoma. Br J Cancer. 2009;100:1647–58. doi: 10.1038/sj.bjc.6605064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation-regulated biquitination and degradation of beta-catenin. J Biol Chem. 1997;272:24735–8. doi: 10.1074/jbc.272.40.24735. [DOI] [PubMed] [Google Scholar]
- Ousingsawat J, Spitzner M, Puntheeranurak S, et al. Expression of voltage-gated potassium channels in human and mouse colonic carcinoma. Clin Cancer Res. 2007;13:824–31. doi: 10.1158/1078-0432.CCR-06-1940. [DOI] [PubMed] [Google Scholar]
- Park C, Chang JY, Hahm ER, et al. Quercetin, a potent inhibitor against β-catenin/Tcf signaling in SW480 colon cancer cells. Biochem Biophys Res Commun. 2005;328:227–34. doi: 10.1016/j.bbrc.2004.12.151. [DOI] [PubMed] [Google Scholar]
- Paul S, Kundu R. Antiproliferative activity of methanolic extracts from two green algae, Enteromorpha intestinalis and Rizoclonium riparium on HeLa cells. DARU. 2013;21:1–12. doi: 10.1186/2008-2231-21-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–8. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Plaumann B, Fritsche M, Rimpler H, Brandner G, Hess RD. Flavonoids activate wild type p53. Oncogene. 1996;13:1605–14. [PubMed] [Google Scholar]
- Puglisi F, Minisini AM, Barbone F, et al. Galectin-3 expression in non-small cell lung carcinoma. Cancer Lett. 2004;212:233–9. doi: 10.1016/j.canlet.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Raftery L, Goldberg RM. Optimal delivery of cytotoxic chemotherapy for colon cancer. Cancer J. 2010;16:214–9. doi: 10.1097/PPO.0b013e3181ddc5ac. [DOI] [PubMed] [Google Scholar]
- Rajamanickam S, Agarwal R. Natural products and colon cancer: current status and future prospects. Drug Dev Res. 2008;69:460–71. doi: 10.1002/ddr.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos S. Cancer chemoprevention and chemotherapy, dietary polyphenols and signalling pathways. Mol Nutr Food Res. 2008;52:507–52. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- Rampone B, Schiavone B, Martino A, Confuorto G. Current role of hyperthermic intraperitoneal chemotherapy in the treatment of peritoneal carcinomatosis from colorectal cancer. World J Gastroenterol. 2010;16:1299–1302. doi: 10.3748/wjg.v16.i11.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Sadik NA, Shaker OG. Inhibitory effect of a standardized pomegranate fruit extract onwnt signalling in 1,2-dimethylhydrazine induced rat colon carcinogenesis. Dig Dis Sci. 2013;58:2507–17. doi: 10.1007/s10620-013-2704-z. [DOI] [PubMed] [Google Scholar]
- Scambia G, Panici PB, Ranelletti FO, et al. Quercetin enhances transforming growth factor beta 1 secretion by human ovarian cancer cells. Int J Cancer. 1994;57:211–5. doi: 10.1002/ijc.2910570214. [DOI] [PubMed] [Google Scholar]
- Seeram NP. Berry fruits for cancer prevention: current status and future prospects. J Agric Food Chem. 2008;55:630–5. doi: 10.1021/jf072504n. [DOI] [PubMed] [Google Scholar]
- Song L, Tang JW, Owusu L, et al. Galectin-3 in cancer. Clin Chim Acta. 2014;431:185–91. doi: 10.1016/j.cca.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Steinberg P. The role of inflammation in colon cancer induced by food contaminants. Toxicology Lett. 2010;196:5–6. [Google Scholar]
- Stevenson DE, Hurst RD. Polyphenolic phytochemicals-just antioxidants or much more? Cell Mol Life Sci. 2007;64:2900–16. doi: 10.1007/s00018-007-7237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner GD. Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prev Res. 2009;2:187–94. doi: 10.1158/1940-6207.CAPR-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin S, Choudhry MA. Quercetin, a bioflavonoid, inhibits the DNA synthesis of human leukemia cells. Biochem Mol Biol Int. 1995;36:545–50. [PubMed] [Google Scholar]
- van Duijnhoven FJ, Bueno-De-Mesquita HB, Ferrari P, et al. Fruit, vegetables, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2009;89:1441–52. doi: 10.3945/ajcn.2008.27120. [DOI] [PubMed] [Google Scholar]
- Vijayababu MR, Arunkumar A, Kanagaraj P, et al. Quercetin downregulates matrix metalloproteinases 2 and 9 proteins expression in prostate cancer cells (PC-3) Mol Cell Biochem. 2006;287:109–16. doi: 10.1007/s11010-005-9085-3. [DOI] [PubMed] [Google Scholar]
- Wada H, Nagano H, Yamamoto H, et al. Combination therapy of interferon alpha and 5-fluorouracil inhibits tumor angiogenesis in human hepatocellular carcinoma cells by regulating vascular endothelial growth factor and angiopoietins. Oncol Rep. 2007;18:801–9. [PubMed] [Google Scholar]
- Yamamoto T, Nagano H, Sakon M, et al. Partial contribution of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)/TRAIL receptor pathway to antitumor effects of interferonalpha/5-fluorouracil against Hepatocellular Carcinoma. Clin Cancer Res. 2004;10:7884–95. doi: 10.1158/1078-0432.CCR-04-0794. [DOI] [PubMed] [Google Scholar]
- Zamin LL, Filippi-Chiela EC, Vargas J, et al. Quercetin promotes glioma growth in a rat model. Food Chem Toxicol. 2014;63:205–11. doi: 10.1016/j.fct.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Zhang N, Ding YQ, Liang L. Association of galectin-3 expression with biological behaviors of human colorectal carcinoma. Nanfang Yikedaxue Xuebao. 2006;26:1685–9. [PubMed] [Google Scholar]
- Zheng Z, Cheng K, Chao J, Wu J, Wang M. Tyrosinase inhibitors from Paper Mulberry (Broussonetia Papyrifera) Food Chem. 2008;106:529–35. [Google Scholar]
- Zou J, Glinsky VV, Landon LA, Matthews L, Deutscher SL. Peptides specific to the galectin-3 carbohydrate recognition domain inhibit metastasis-associated cancer cell adhesion. Carcinogenesis. 2005;26:309–18. doi: 10.1093/carcin/bgh329. [DOI] [PubMed] [Google Scholar]


