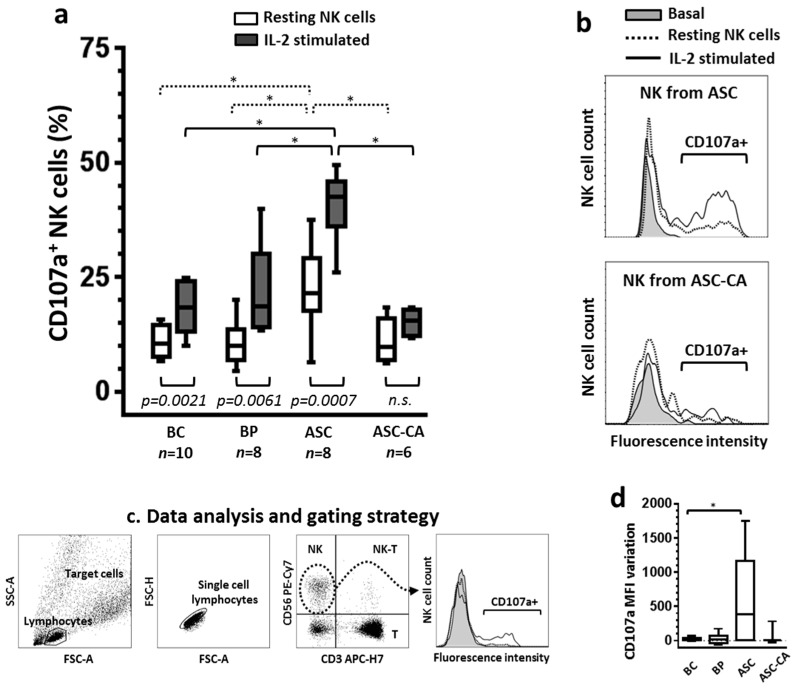
Figure 1.
(a) Comparison of degranulation between resting and IL-2 stimulated natural killer (NK) cells from blood control (BC), blood of patients with advanced ovarian cancer (BP), epithelial ovarian cancer (EOC) cell-free ascites (ASC) and ascites with EOC cells (ASC-CA). Degranulation was evaluated by the expression of the CD107a molecule on NK cells, resting and after IL-2 stimulation overnight, while coincubated (2 h, ratio 1:1) with K562 target cells. Overnight stimulation with rhIL-2 (1000 UI/mL) was conducted in RPMI-1640 medium supplemented with FBS (10%) and l-glutamine (2 mM). Values are presented in whisker plots as medians; (b) Histograms are representative of the CD107a fluorescence intensity profiles of NK cells from ASC and ASC-CA and, the fluorescence intensity levels of the samples were the closest to the mean of the group represented. Basal curve indicates the “background” expression of CD107a of resting NK cells in the absence of target cells K562; (c) Flow cytometry-based analysis of NK cell degranulation. To determine CD107a expression, NK cells were gated from the whole lymphocyte population, based on their expression of CD56 molecule and absence of CD3; (d) Variation of the mean fluorescence intensity (MFI) was calculated by subtracting CD107a MFI of resting NK cells from CD107a MFI of IL-2 stimulated NK cells. Statistical analyses within groups were performed by Student’s t-test for dependent samples; among groups by ANOVA followed by Tukey’s multiple comparisons test, and p-values (* p < 0.05 on the brackets) indicate significant statistical differences.