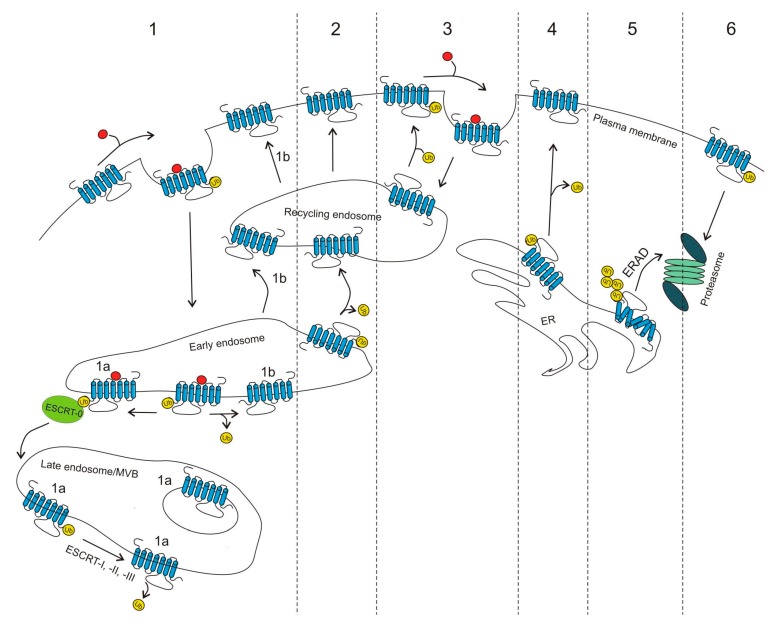
Figure 1.
Role of ubiquitin in GPCR trafficking. (1) Many GPCRs have been described to undergo agonist-induced ubiquitination and down-regulation. Upon endocytosis, they are often directed for lysosomal degradation via the conserved endosomal-sorting complex required for the transport (ESCRT) machinery (1a); however, some of them can be deubiquitinated and directed to the resensitization pathway (1b). (2) Constitutive ubiquitination of frizzled-4 receptor (FZD4R) promotes its internalization and lysosomal degradation, while deubiquitination leads to its recycling and increased cell surface expression. (3) Some GPCRs are basally ubiquitinated (steady-state) and upon agonist binding are deubiquitinated and internalized. After subsequent ubiquitination, they can recycle back to the cell surface. (4) Some properly folded GPCRs (e.g., A2AR) require deubiquitination to be delivered to the cell surface. (5) Ubiquitination also functions as a quality control system in which misfolded, polyubiquitinated receptors are directed for proteasomal degradation via the endoplasmic reticulum-associated degradation (ERAD) pathway. (6) Some GPCRs are ubiquitinated at the plasma membrane and are directed to the proteasome via a poorly understood process.