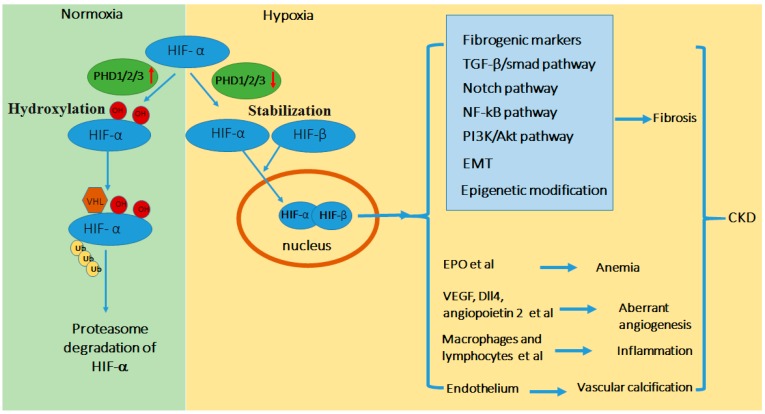
Figure 1.
Schematic diagram of HIF regulation in CKD. In normoxia or the presence of O2, HIF-α is hydroxylated by prolyl hydroxylase domain (PHD) resulting in its binding of von Hippel Lindau protein (pVHL)-E3-ubiquitin ligase complex, poly-ubiquitination, and consequent proteosomal degradation. In hypoxia or the absence of O2, PHD-mediated hydroxylation is inhibited, leading to HIF-α stabilization and accumulation. HIF-α dimerizes with HIF-β to form functional HIF that translocates to nucleus to activate down-stream gene transcription. Hypoxia is a feature in kidney tissues in CKD that activates HIF, which integrates multiple signaling networks to induce renal fibrosis. In addition, tissue hypoxia and HIF contribute to other CKD-associated pathogenic processes including anemia, angiogenesis, inflammation, and vascular calcification. Abbreviations: Epithelial-Meshenchymal Transition (EMT), Erythropoietin (EPO), vascular endothelial growth factor (VEGF), transforming growth factor (TGF).