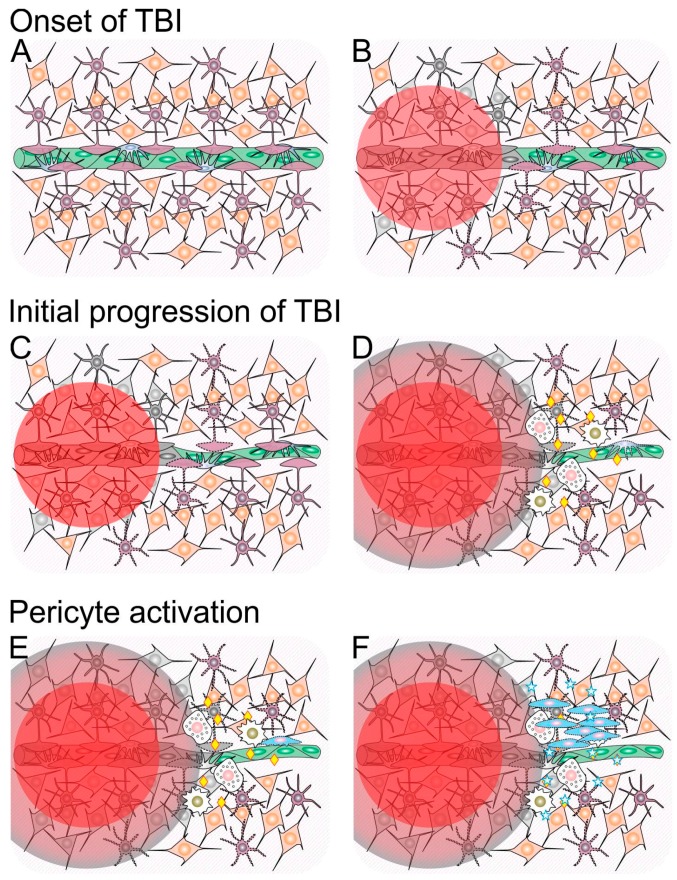
Figure 1.
Schematic representation of cellular and molecular events involved in the progression and resolution of traumatic brain injury (TBI). (A) Integer neural tissue composed of neurons (orange) and astrocytes (purple) nearby a blood capillary composed of endothelial cells (green) and pericytes (blue). For the sake of simplification, other cell types present in the neural tissue (e.g., oligodendrocytes, microglial cells) and the basement membrane that surrounds the capillary are not shown; (B) TBI causes a primary neural injury, represented by a red circle, which leads to cell death (represented by color change to gray); (C) Glial cells surrounding the primary lesion site become activated (represented by a change from a solid to a dotted outline), and vasoconstriction takes place; (D) Within a few hours following TBI, dying cells in the primary lesion site release a number of toxic molecules that diffuse to the surroundings (penumbra represented in red-to-gray gradient), which causes further cell death and glial cell activation. Concomitantly, the blood–brain barrier is disrupted (represented by loss of coverage of the capillary by astrocyte feet), and innate immune system cells (white), including monocytes/macrophages (golden nuclei) and neutrophils (pink nuclei), reach the site and release a number of inflammatory signaling molecules (yellow diamonds); (E) During the first day after TBI, inflammation increases and is accompanied by pericyte activation (represented by a change from a solid to a dotted outline); (F) Between one and three days after TBI, activated pericytes leave their native perivascular niche, proliferate and secrete a number of antiapoptotic, trophic and immunomodulatory molecules (represented as blue stars) that counteract cell death and inflammation triggered by TBI.