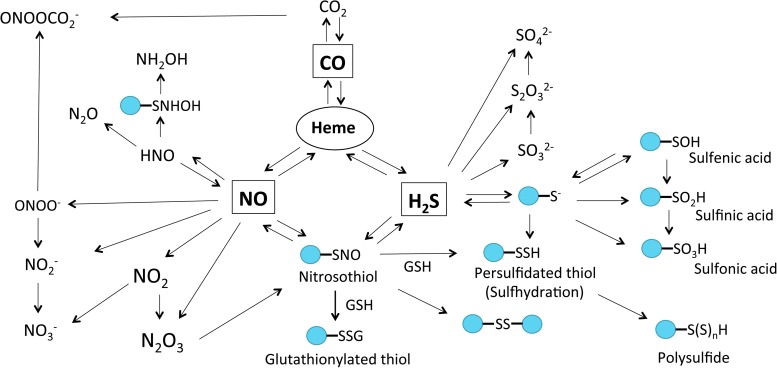
FIG. 3.
Molecular target interactions and metabolic products of CO, NO, and H2S. All gasotransmitters are capable of directly interacting with heme. Reaction of NO and sulfide metabolites can lead to nitrosothiol (RSNO) formation, which can also be produced from the reaction of dinitrogen trioxide (N2O3) and thiol. RSNO is reactive nitrogen species that can react with GSH or thiol, resulting in the production of glutathionylated thiol (RSSG), disulfide bonds (RS-SR), or sulhydrated thiol [PSSH, PS-(S)nH], respectively. CO, NO, and H2S also can be oxidized to form dinitrogen trioxide (N2O3), nitrogen dioxide (NO2), nitrite (NO2−), peroxynitrite (ONOO−), HNO, carbon dioxide (CO2), sulfenic acid (R-SOH), sulfinic acid (R-SO2H), sulfonic acid (R-SO3H), sulfite (SO32−), and sulfate (SO42−), respectively. R-SOH can be directly reduced to free thiol by Trx or further oxidized to generate R-SO2H or R-SO3H. In mitochondria, SQR can use GSH as an acceptor of sulfide and form glutathione persulfide (GSSH), which can be converted to thiosulfate (S2O32−) by rhodanese. HNO can quickly dimerize to hyponitrous acid (H2N2O2), which is then dehydrated to nitrous oxide (N2O). HNO can also generate hydroxylamine ammonia (NH2OH). GSH, glutathione; HNO, nitroxyl; RSNO, protein S-nitrosothiol; SQR, sulfide–quinone oxidoreductase.