Abstract
Postsepsis lung injury is a common clinical problem associated with significant morbidity and mortality. Leukotrienes (LTs) are important lipid mediators of infection and inflammation derived from the 5-lipoxygenase (5-LO) metabolism of arachidonate with the potential to contribute to lung damage after sepsis. To test the hypothesis that LTs are mediators of lung injury after sepsis, we assessed lung structure, inflammatory mediators, and mechanical changes after cecal ligation and puncture surgery in wild-type (WT) and 5-LO knockout (5-LO−/−) mice and in WT mice treated with a pharmacologic LT synthesis inhibitor (MK886) and LT receptor antagonists (CP105,696 and montelukast). Sixteen hours after surgery, WT animals exhibited severe lung injury (by histological analysis), substantial mechanical impairment (i.e., an increase in static lung elastance), an increase in neutrophil infiltration, and high levels of LTB4, cysteinyl-LTs (cys-LTs), prostaglandin E2, IL-1β, IL-6, IL-10, IL-17, KC (CXCL1), and monocyte chemotactic protein–1 (CCL2) in lung tissue and plasma. 5-LO−/− mice and WT mice treated with a pharmacologic 5-LO inhibitor were significantly protected from lung inflammation and injury. Selective antagonists for BLT1 or cys-LT1, the high-affinity receptors for LTB4 and cys-LTs, respectively, were insufficient to provide protection when used alone. These results point to an important role for 5-LO products in sepsis-induced lung injury and suggest that the use of 5-LO inhibitors may be of therapeutic benefit clinically.
Keywords: 5-lipoxygenase, cytokines, leukotrienes, lung injury, sepsis
Clinical Relevance
Acute lung injury after sepsis is a common clinical problem associated with significant morbidity and mortality. Here we provide evidence that 5-lipoxygenase products are involved in the injury observed, and we suggest that 5-lipoxygenase inhibition could have beneficial effects when its use is carefully considered.
Sepsis leading to shock and organ failure is associated with high rates of mortality and morbidity and enormous health care costs (1). Sepsis is the third leading cause of death and is responsible for almost 10% of deaths in the United States (2). Management of patients with sepsis is largely limited to supportive therapies, reflecting an incomplete understanding of the underlying pathophysiology. Intestinal perforation and contamination of the abdominal cavity with microbes and toxins stimulate local formation of numerous proinflammatory compounds, which subsequently diffuse into the systemic circulation (3). The lung is the most sensitive and clinically important end organ for the systemic inflammatory response in abdominal sepsis (4).
Leukotrienes (LTs) are lipid mediators produced from the 5-lipoxygenase (5-LO) metabolism of arachidonic acid. The 5-LO enzyme, together with 5-LO activating protein (FLAP), oxygenates the C5 carbon of arachidonic acid to yield LTA4. This unstable intermediate can be hydrolyzed to LTB4, a potent leukocyte chemoattractant and activator, or can be conjugated with glutathione to form LTC4, which is then metabolized extracellularly to LTD4 and LTE4. Collectively known as cysteinyl-LTs (cys-LTs), these are important inducers of smooth muscle contraction and vascular permeability (5). Both classes of LTs play important roles in host defense against a variety of infectious agents, in part by enhancing phagocytosis and the killing capacity of leukocytes (6). We have previously reported that 5-LO knockout (5-LO−/−) mice exhibit an impaired clearance and higher susceptibility to intrapulmonary Klebsiella pneumonia challenge (7). In a cecal ligation and puncture (CLP) model of peritonitis with severe sepsis, 5-LO−/− mice showed a decrease in peritoneal neutrophil recruitment and an increase in the number of bacteria recovered from the peritoneal cavity. Despite this impairment of local innate immunity, the null mice exhibited a marked improvement in survival. This protection was also seen in wild-type (WT) animals treated with the LT synthesis inhibitor MK886 (8).
Several reports in the literature suggest that the 5-LO pathway is important in the development of lung injury induced by hemorrhagic shock, hyperoxia, LPS, mechanical ventilation, and ischemia-reperfusion (9–13); however, no information is available regarding its role in microbial sepsis-induced lung injury. In the current study, we used 5-LO−/− mice and pharmacologic tools to evaluate the role of 5-LO products in sepsis-induced lung injury, analyzing lung structure and function as well as local and systemic inflammation.
Materials and Methods
Animals and Protocol
This study was approved by the Ethics Committee of the Carlos Chagas Filho Institute of Biophysics, Federal University of Rio de Janeiro (IBCCF019), and performed in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals from the National Academy of Sciences.
Pathogen-free 5-LO−/− (129-Alox5) and strain-matched WT mice and C57Bl/6 mice were bred in the Laboratory of Transgenic Animals (Federal University of Rio de Janeiro) from breeders from The Jackson Laboratory. Animals (18–20 g) were subjected to CLP surgery as previously described (14). After 16 hours, the animals were anesthetized for evaluation of respiratory mechanics; the animals were killed, and lungs were prepared for histologic analysis and enzymatic activity; and blood and lungs were collected for quantification of mediators.
Pharmacologic Treatments
MK886 (BIOMOL, Plymouth, PA) was orally administered (1 mg/kg) 1 hour before CLP. Montelukast (Cayman Chemicals, Ann Arbor, MI) was administered (1 mg/kg, subcutaneously) 4 hours before and 4 hours after surgery. CP105,696 (a gift from Pfizer, Groton, CT) was administered (3 mg/kg, subcutaneously) 4 hours before and 4 hours after surgery.
Respiratory Mechanics
Animals were sedated with diazepam (1 mg/kg, intraperitoneally), anesthetized with thiopental sodium (20 mg/kg, intraperitoneally), tracheotomized, paralyzed with vecuronium bromide (0.005 mg/kg, intravenously), and ventilated with a constant flow ventilator (Samay VR15; Montevideo, Uruguay) with 100 breaths/min frequency, tidal volume of 0.2 ml, and fraction of inspired oxygen of 0.21. The anterior chest wall was removed, and a positive end-expiratory pressure of 2 cm H2O was applied. After 10 minutes, lung mechanics were computed. Airflow and tracheal pressure were measured (15). Static lung elastance was computed by the end-inflation occlusion method (16). Mechanics measurements were performed 10 times- per animal. Data were analyzed using ANADAT software (RHT-InfoData, Inc., Montreal, PQ, Canada).
Lung Histology
After lung mechanics, heparin (1,000 IU) was injected intravenously. The trachea was clamped at end-expiration (positive end-expiratory pressure, 2 cm H2O), and mice were exsanguinated. The right lung was removed, fixed in 3% buffered formaldehyde, and paraffin embedded. Slices (4 μm thick) were stained with hematoxylin and eosin. Lung morphometry analysis was performed as previously described (17, 18).
Myeloperoxidase Activity
Myeloperoxidase (MPO) activity was determined as previously described (19).
Cytokines, LTB4, cys-LTs, and PGE2 Analysis
Right lung tissue was placed in 400 μl of cold NaCl/Tris buffer (50 mM/100 mM [pH 8]) with protease inhibitors (Sigma, St Louis, MO), homogenized, and centrifuged (590 g for 5 min at 4°C), and supernatants were stored (−80°C) for cytokine and LTB4 measurement. Plasma was stored (−80°C) for cytokine measurement.
Cytokines were determined by ELISA (BD, Franklin Lakes, NJ), and LTB4, cys-LTs, and PGE2 were determined by enzyme immunoassay (Cayman Chemicals) according to the manufacturer’s instructions.
Statistical Analysis
Data are presented as mean ± SEM. Statistical analysis was determined by ANOVA with Bonferroni t test for unpaired values or Student t test as appropriate. Tests were performed using the SPSS version 18.0 (SPSS, Chicago, IL), and significance was set as P < 0.05. The results from the experiment are representative of three independent experiments, with six mice per group in each experiment.
Results
Pulmonary Histopathologic Protection in 5-LO−/− Mice after CLP
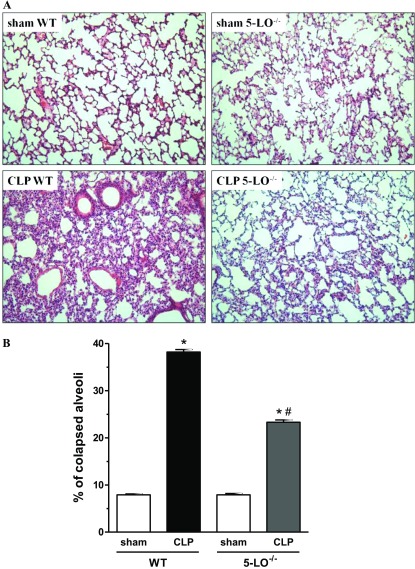
Lungs from CLP/WT mice exhibited a high degree of inflammatory injury, with cellular accumulation noted predominantly in the interstitium and thickening and edema of the alveolar walls as compared with lungs from sham-operated mice (Figure 1A). CLP/5-LO−/− mice exhibited much less injury and inflammation than CLP/WT animals (Figure 1A). Lung structures of sham-operated WT and 5-LO−/− mice were similar without any evidence of damage. Morphometric analysis demonstrated that sepsis induced collapse of almost 40% of alveoli in WT animals but induced a collapse of only 23.3% in 5-LO−/− mice. The percentage of collapsed alveoli in the lungs of sham-operated groups was similar in WT and 5-LO−/− mice (Figure 1B).
Figure 1.
Morphologic changes in sham and cecal ligation and puncture (CLP) wild-type (WT) (sv129 strain) and 5-lipoxygenase knockout (5-LO−/−) mice. (A) Representative lung sections from sham/WT (upper left), CLP/WT (lower left), sham/5-LO−/− (upper right), and CLP/5-LO−/− mice (lower right) obtained 16 hours after surgery and stained with hematoxylin and eosin. (B) Morphometry of sham and CLP WT and 5-LO−/− mice. Lungs were collected 16 hours after surgery and prepared for histology. Data are presented as the mean ± SEM. *P < 0.05 compared with sham group. #P < 0.05 compared with CLP WT mice.
LT Levels and MPO Activity in the Lungs after CLP
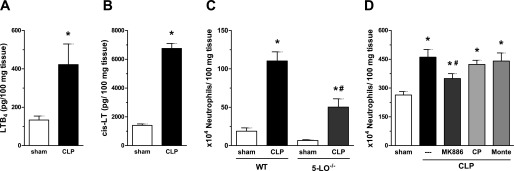
Higher amounts of LTB4 (Figure 2A), cys-LTs (Figure 2B), and PGE2 (see Figure E1 in the online supplement) were detected in lung tissue of WT animals subjected to CLP compared with sham mice. Having determined that LTs were generated in the lungs during sepsis, we evaluated its contribution to neutrophil accumulation, assessed by MPO activity. WT (sv129) mice subjected to CLP showed higher neutrophil accumulation in the lungs compared with sham-operated animals (Figure 2C). However, CLP-induced neutrophil migration to the lungs was significantly attenuated in 5-LO−/− mice (Figure 2C). This reduction in the numbers of neutrophils was not associated with reduced numbers of neutrophils in peripheral circulation or bone marrow of 5-LO−/− mice because they present similar numbers to those observed in WT (sv129) mice (data not shown). Corroborating this finding, the treatment of C57Bl/6 mice with a FLAP inhibitor (MK886) reduced the number of infiltrating neutrophils in the lungs of animals subjected to CLP (Figure 2C). Receptor antagonism of the high-affinity LTB4 receptor BLT1 (CP105,696) or the high-affinity cysteinyl receptor cys-LT1 (montelukast), in vivo had no such effect (Figure 2D).
Figure 2.
Leukotriene (LT) production and neutrophil influx into the lung after CLP. Concentrations of LTB4 (A) and cys-LTs (B) in lung homogenates of C57Bl/6 mice were obtained 16 hours after sham, and CLP was determined by enzyme immunoassay. Neutrophil infiltration in WT and 5-LO−/− mice (C) and MK886-, CP 105,696–, and montelukast-treated mice lungs (D) was evaluated 16 hours post-sham or CLP by MPO activity. Data are presented as the mean ± SEM. *P < 0.05 compared with sham group. #P < 0.05 compared with CLP/WT mice.
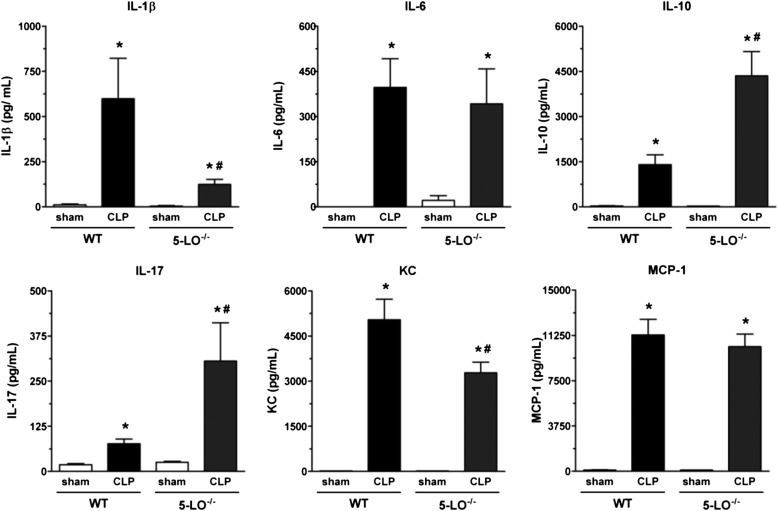
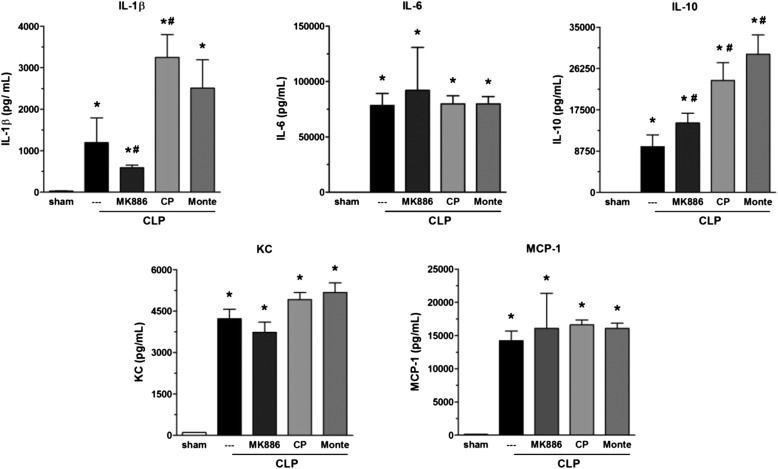
CLP-Induced Lung Cytokines: Effect of 5-LO Deletion/Inhibition and Receptor Antagonists
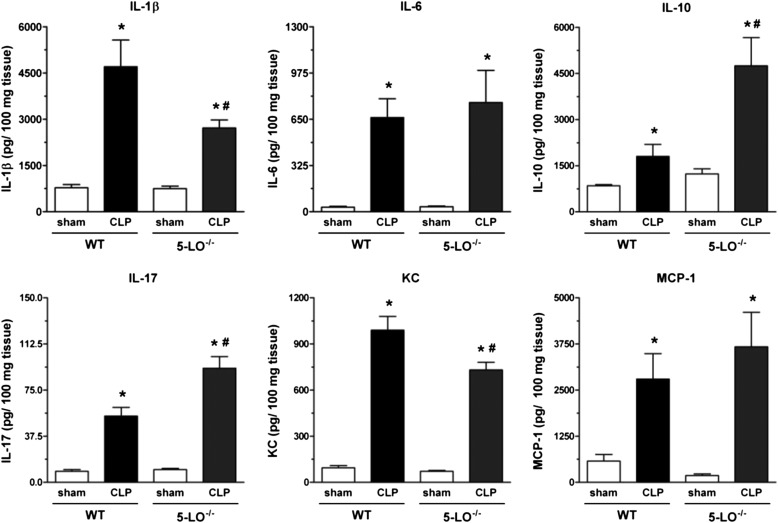
Ample precedent exists for generation of cytokines/chemokines to be influenced by 5-LO pathway products (20–24). Lungs from CLP/WT mice exhibited high quantities of IL-1β, IL-6, IL-10, IL-17, KC, and MCP-1 as compared with sham-operated mice. Lungs from CLP/5-LO−/− mice showed a reduction in levels of IL-1β and KC, no difference in IL-6 and MCP-1, and a significant increase in IL-17 and IL-10 compared with the CLP/WT group (Figure 3). All values observed in sham-operated groups (WT and 5-LO−/−) were similar. A similar effect was obtained with MK886, with a decrease in IL-1β and an increase in IL-10. No differences were noted for IL-6, KC, and MCP-1 when compared with untreated mice (Figure 4). The BLT1 and cys-LT1 antagonists had no effect on CLP-induced lung IL-6, KC, and MCP-1. However, both antagonists enhanced IL-1β and IL-10 concentrations after CLP surgery (Figure 4).
Figure 3.
Lung cytokine levels in WT (sv129 strain) and 5-LO−/− mice subjected to sham or CLP. IL-1β, IL-6, IL-10, IL-17, KC, and MCP-1 levels were quantified in lungs 16 hours after sham or CLP of WT and 5-LO−/− mice. Cytokines were measured by ELISA. Data are presented as the mean ± SEM. *P < 0.05 compared with sham group. #P < 0.05 compared with CLP/WT group.
Figure 4.
Effect of MK886, BLT1, and cys-LT1 receptor antagonists on lung cytokine levels after CLP. IL-1β, IL-6, IL-10, KC, and MCP-1 levels were quantified in lungs of C57Bl/6 mice 16 hours after sham or CLP in MK886-, CP105,696 (CP)-, or montelukast (Monte)-treated or nontreated mice. Cytokines were measured by ELISA. Data are presented as the mean ± SEM. *P < 0.05 compared with sham group. #P < 0.05 compared with CLP untreated mice.
CLP-Induced Systemic Cytokines: Effect of 5-LO Deletion/Inhibition and Receptor Antagonists
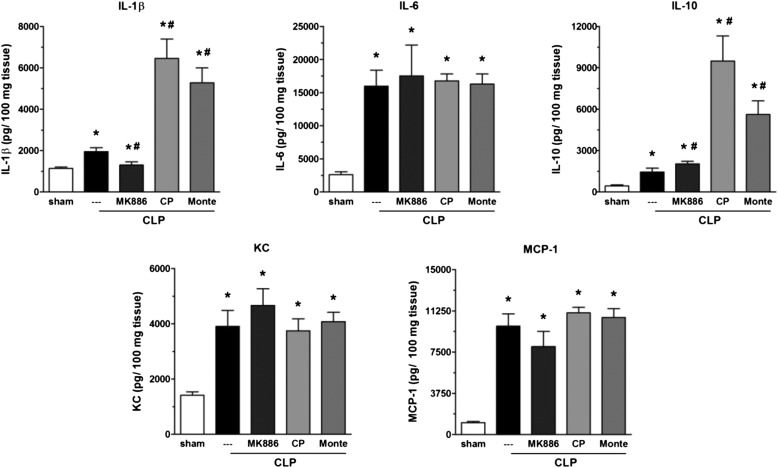
IL-1β, IL-6, IL-10, IL-17, KC, and MCP-1 levels in plasma from CLP animals were elevated when compared with those obtained from sham-operated animals. As observed in lung tissue, CLP/5-LO−/− mice exhibited a reduction in IL-1β and KC plasma levels, no change in IL-6 and MCP-1, and increased IL-10 and IL-17 compared with the CLP/WT group (Figure 5). All cytokines analyzed were very low or undetectable in the plasma of sham-operated mice. MK886 treatment also reduced IL-1β and enhanced IL-10 plasma levels compared with the CLP group but had no effect on the other cytokines analyzed (Figure 6). Treatment of CLP mice with CP 105,696 or montelukast evoked an increase in IL-1β and IL-10 when compared with the CLP group without affecting the levels of the other cytokines measured (Figure 6).
Figure 5.
Plasma cytokine levels after sepsis in WT (sv129 strain) and 5-LO−/− mice. IL-1β, IL-6, IL-10, IL-17, KC, and MCP-1 levels were quantified in plasma 16 hours after sham or CLP in WT and 5-LO−/− mice by ELISA. Data are presented as the mean ± SEM. *P < 0.05 compared with sham group. #P < 0.05 compared with CLP/WT mice.
Figure 6.
Effects of MK886, BLT1, and cys-LT1 receptor antagonists on plasma cytokine levels after sepsis. IL-1β, IL-6, IL-10, KC, and MCP-1 levels were quantified in lungs 16 hours after sham or CLP in untreated or treated C57Bl/6 mice with MK886, CP105,696 (CP), or montelukast (Monte) by ELISA. Data are presented as the mean ± SEM. *P < 0.05 compared with sham group. #P < 0.05 compared with CLP untreated mice.
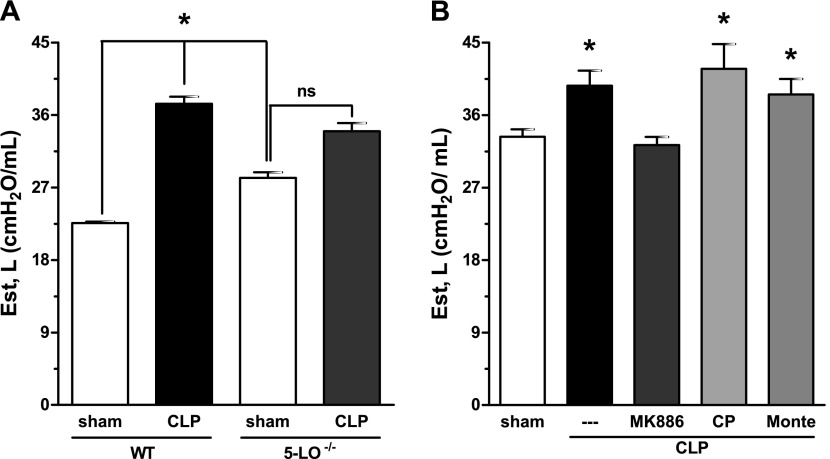
Lung Physiologic Protection in 5-LO−/− Mice and in MK886-Treated Animals
CLP/WT mice showed a substantial (63.5%) increase in lung elastance compared with sham-operated animals, consistent with the decrease in compliance during acute respiratory distress syndrome. CLP/5-LO−/− mice exhibited a smaller (21%) increase in lung elastance (Figure 7A). Basal levels of elastance in sham-operated 5-LO−/− mice were modestly higher than those in the control group (WT). The same difference in elastance between WT and 5-LO−/− animals was observed in naive mice (data not shown). Next, we evaluated lung elastance in mice treated with LT synthesis inhibitor (MK886), BLT1 (CP105696), and cys-LT1 (montelukast) receptor antagonists subjected to CLP. CLP-induced lung elastance increase was only inhibited by MK886 treatment and was not altered by CP105696 or montelukast (Figure 7B).
Figure 7.
Lung function in 5-LO−/− mice and MK886-treated animals after CLP. Static elastance (Est) was evaluated 16 hours after sham or CLP in WT (sv129 strain) and 5-LO−/− mice (A) and in untreated or treated C57Bl/6 mice with MK886, CP105,696 (CP), or montelukast (Monte) (B). Data are presented as the mean ± SEM of six animals per group. *P < 0.05 compared with sham group. ns = nonsignificant.
Discussion
Lung injury can occur by direct insults, such as infections, inhalation of toxins, hyperoxia, or aspiration of gastric contents. However, it is also often associated with systemic inflammatory responses, such as burns, trauma, pancreatitis, hemorrhagic shock, and sepsis (25). Lung injury is a severe consequence of sepsis and is one of the most common causes of acute respiratory distress syndrome. Cytokines are well known participants in the inflammatory injury to organs including the lung during sepsis. Although the 5-LO pathway has been implicated in lung injury, including that elicited by LPS administration (26), its role in sepsis-induced lung injury has not previously been examined. Recently, a study demonstrated that a dual inhibitor of cyclooxygenase 2 and 5-LO attenuates the inflammatory response and improves survival in murine CLP, but the independent contributions of these two enzymatic pathways were not distinguished (27). Moreover, no previous studies have determined the role of 5-LO products in the physiologic derangements associated with acute lung injury (ALI). Our data demonstrate that the morphologic, physiologic, and inflammatory features of the lung injury induced by CLP, a relevant animal model of sepsis, are at least in part attributable to 5-LO products. The results also point to an important role for 5-LO–derived mediators as key modulators of cytokine production in this setting.
CLP-induced lung injury depends on 5-LO–derived mediators. In our study, 5-LO−/− mice showed less morphologic damage and a corresponding protection from increased lung elastance. Further evidence of 5-LO pathway participation was obtained with pretreatment of WT animals with a FLAP inhibitor (MK886), which resulted in less neutrophil recruitment to the lungs and a decrease in IL-1β and KC levels in the lung and plasma and inhibited CLP-induced lung elastance increase. IL-1β and KC are important contributors to neutrophil migration to inflammatory sites (28, 29), and we have previously reported that neutrophil accumulation induced by IL-1β depends on LTB4 production (24). The importance of IL-1β is emphasized by a recent report highlighting the critical role of inflammasome-regulated cytokines in ventilation-induced ALI in humans (30). Interventions that reduce IL-1β in ALI may be beneficial to the host. It was also recently demonstrated that inflammasome activation can elicit the rapid production of prostaglandins and LTs in vivo independently of IL-1β and IL-18 (31). Genetic and pharmacologic disruption of LT biosynthesis led to an increase in IL-10 levels in plasma and in the lungs, and this represents an additional possible protective mechanism because the antiinflammatory actions of IL-10 are well established (32, 33). We also observed a higher production of IL-17 in the lungs and plasma of 5-LO−/− animals subjected to CLP (Figures 4 and 5). There is evidence in the literature that IL-1β can act as an important inducer of Th 17 cells (34, 35), so it was surprising that IL-17 and IL-1β levels tended to be regulated in opposing fashion by 5-LO deletion/inhibition. Although 5-LO−/− mice exhibited higher IL-17 production and IL-17 promotes neutrophil recruitment, we observed a reduction in neutrophil infiltration into lungs in this setting, which may be due to concomitant reductions in IL-1β and KC secondary to reduced LTB4. IL-17 is involved in autoimmune diseases but has emerged as a critical player in host defense responses and inflammatory diseases (36, 37). It is possible that higher IL-17 levels represent a compensatory effort to better control the infection during polymicrobial sepsis, as previously suggested (38).
Neither genetic nor pharmacologic disruption of LT biosynthesis distinguishes the potential roles of LTB4, cys-LTs, and other 5-LO metabolites. We therefore treated the animals with selective antagonists for BLT1 and cys-LT1 to evaluate their individual contributions to cytokine modulation. Both of these treatments led to an increase in IL-1β and IL-10 production. These results contrast with those obtained by targeting the biosynthetic 5-LO–FLAP step and point to complex actions and interplay between BLT1 and cys-LT1 receptors. Furthermore, the possible participation of other 5-LO products (e.g., 5-HETE), not evaluated in this study, is not ruled out. Although our results suggest that 5-LO products participate in the pathogenesis of sepsis-induced lung injury, specific LT receptor blockade was substantially less protective than 5-LO inhibition. Several possible explanations exist for this discrepancy. First, the 5-LO and cyclooxygenase enzymes can compete for their common substrate, arachidonic acid. Shunting of arachidonic acid through the cyclooxygenase pathway leading to an increased production of PGE2 has been observed in macrophages from 5-LO−/− mice (39, 40), and increased lung lavage PGE2 levels have been reported in 5-LO−/− mice (41). PGE2 is able to inhibit leukocyte recruitment (42) and the production of IL-8 (43) and TNF-α (44) and to promote IL-10 synthesis (45). Another possible explanation relates to the fact that the antagonists used specifically antagonize the high-affinity receptors BLT1 and cys-LT1 but not the low-affinity receptors BLT2 and cys-LT2. These findings are in accordance with our previous report (8), in which resistance against CLP-induced mortality was observed in 5-LO−/− mice or WT animals treated with the FLAP inhibitor (MK886) but not in animals treated with a BLT1 antagonist. In that study, a cys-LT1 antagonist conferred a moderate degree of protection against lethality, which was attributed to less sepsis-induced hypotension. Furthermore, the data presented here and elsewhere (8) support that, independent of the mouse strain used (sv129 or C57Bl/6), we were able to observe relevant protective effects during 5-LO inhibition or deletion but not with the use of receptor antagonists alone.
The increased baseline elastance in 5-LO−/− mice may represent an intrinsic characteristic of 5-LO−/− lungs. Differences in quantities and/or types of matrix proteins may exist, but this requires further characterization. Nevertheless, 5-LO−/− mice and MK886-treated mice were clearly protected from further elevation in elastance resulting from CLP.
In conclusion, our data suggest that 5-LO products contribute to sepsis-induced lung injury, and we speculate that 5-LO inhibition could be beneficial as a strategy to dampen inflammatory responses observed after sepsis. However, 5-LO products promote innate immune responses, and blocking this pathway could interfere with host defense. For this reason, the use and timing of 5-LO inhibitors to impair sepsis-induced lung injury must be carefully considered.
Acknowledgments
Acknowledgments
The authors thank Heloísa Lopes dos Santos, Milena Vasconcellos de Oliveira, and Raquel Ferreira de Magalhães for technical assistance and lung mechanics analysisand Pfizer for the gift of CP 105696.
Footnotes
This work was supported by the Carlos Chagas Filho Rio de Janeiro State Research Supporting Foundation (FAPERJ), The Brazilian Council for Scientific and Technological Development (CNPq), José Bonifácio Foundation (FUJB), the Centers of Excellence Program (PRONEX-FAPERJ), The National Institute of Science and Technology of Drugs and Medicine (INCT-INOFAR), and Coordination for the Improvement of Higher Level Personnel (CAPES).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0525OC on August 15, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.O’Brien JM, Jr, Ali NA, Aberegg SK, Abraham E. Sepsis. Am J Med. 2007;120:1012–1022. doi: 10.1016/j.amjmed.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenstern C, Brenner T, Bardenheuer HJ, Weigand MA. Predictors of survival in sepsis: what is the best inflammatory marker to measure? Curr Opin Infect Dis. 2012;25:328–336. doi: 10.1097/QCO.0b013e3283522038. [DOI] [PubMed] [Google Scholar]
- 4.Sadowitz B, Roy S, Gatto LA, Habashi N, Nieman G. Lung injury induced by sepsis: lessons learned from large animal models and future directions for treatment. Expert Rev Anti Infect Ther. 2011;9:1169–1178. doi: 10.1586/eri.11.141. [DOI] [PubMed] [Google Scholar]
- 5.Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway: biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990;323:645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- 6.Peters-Golden M, Coffey M. Role of leukotrienes in antimicrobial defense of the lung. J Lab Clin Med. 1998;132:251–257. doi: 10.1016/s0022-2143(98)90037-3. [DOI] [PubMed] [Google Scholar]
- 7.Bailie MB, Standiford TJ, Laichalk LL, Coffey MJ, Strieter R, Peters-Golden M. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumonia in association with decreased alveolar macrophage phagocytic and bactericidal activities. J Immunol. 1996;157:5221–5224. [PubMed] [Google Scholar]
- 8.Benjamim CF, Canetti C, Cunha FQ, Kunkel SL, Peters-Golden M. Opposing and hierarchical roles of leukotrienes in local innate immune versus vascular responses in a model of sepsis. J Immunol. 2005;174:1616–1620. doi: 10.4049/jimmunol.174.3.1616. [DOI] [PubMed] [Google Scholar]
- 9.Ishizaka A, Hasegawa N, Sakamaki F, Tasaka S, Nakamura H, Kishikawa K, Yamada A, Obata T, Sayama K, Urano T, et al. Effects of ONO-1078, a peptide leukotriene antagonist, on endotoxin-induced acute lung injury. Am J Respir Crit Care Med. 1994;150:1325–1331. doi: 10.1164/ajrccm.150.5.7952560. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura K, Nakagawa S, Koyama S, Kobayashi T, Homma T. Roles of neutrophil elastase and superoxide anion in leukotriene B4-induced lung injury in rabbit. J Appl Physiol. 1994;76:91–96. doi: 10.1152/jappl.1994.76.1.91. [DOI] [PubMed] [Google Scholar]
- 11.Phillips GJ, Mohammed W, Kelly FJ. Oxygen-induced lung injury in the pre-term guinea pig: the role of leukotriene B4. Respir Med. 1995;89:607–613. doi: 10.1016/0954-6111(95)90228-7. [DOI] [PubMed] [Google Scholar]
- 12.VanderMeer TJ, Menconi MJ, O’Sullivan BP, Larkin VA, Wang H, Sofia M, Fink MP. Acute lung injury in endotoxemic pigs: role of leukotriene B4. J Appl Physiol. 1995;78:1121–1131. doi: 10.1152/jappl.1995.78.3.1121. [DOI] [PubMed] [Google Scholar]
- 13.Furue S, Kuwabara K, Mikawa K, Nishina K, Shiga M, Maekawa N, Ueno M, Chikazawa Y, Ono T, Hori Y, et al. Crucial role of group IIA phospholipase A(2) in oleic acid-induced acute lung injury in rabbits. Am J Respir Crit Care Med. 1999;160:1292–1302. doi: 10.1164/ajrccm.160.4.9812042. [DOI] [PubMed] [Google Scholar]
- 14.Baker CC, Chaudry IH, Gaines HO, Baue AE. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 15.Ornellas DS, Maron-Gutierrez T, Ornellas FM, Cruz FF, Oliveira GP, Lucas IH, Fujisaki L, Oliveira MG, Teodoro WR, Capelozzi VL, et al. Early and late effects of bone marrow-derived mononuclear cell therapy on lung and distal organs in experimental sepsis. Respir Physiol Neurobiol 2011. 178:304–314. [DOI] [PubMed]
- 16.Bates JH, Ludwig MS, Sly PD, Brown K, Martin JG, Fredberg JJ. Interrupter resistance elucidated by alveolar pressure measurement in open-chest normal dogs. J Appl Physiol. 1988;65:408–414. doi: 10.1152/jappl.1988.65.1.408. [DOI] [PubMed] [Google Scholar]
- 17.Hsia CC, Hyde DM, Ochs M, Weibel ER. How to measure lung structure—what for? On the “Standards for the quantitative assessment of lung structure.”. Respir Physiol Neurobiol. 2010;171:72–74. doi: 10.1016/j.resp.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Rocco PR, Negri EM, Kurtz PM, Vasconcellos FP, Silva GH, Capelozzi VL, Romero PV, Zin WA. Lung tissue mechanics and extracellular matrix remodeling in acute lung injury. Am J Respir Crit Care Med. 2001;164:1067–1071. doi: 10.1164/ajrccm.164.6.2007062. [DOI] [PubMed] [Google Scholar]
- 19.Moreno SE, Alves-Filho JC, Bertozi G, Alfaya TM, Thèze J, Ferreira SH, Vargaftig BB. Systemic administration of interleukin-2 inhibits inflammatory neutrophil migration: role of nitric oxide. Br J Pharmacol. 2006;148:1060–1066. doi: 10.1038/sj.bjp.0706835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remick DG, Larrick JW, Nguyen DT, Kunkel SL. Stimulation of prostaglandin E2 and thromboxane B2 production by human monocytes in response to interleukin-2. Biochem Biophys Res Commun. 1987;147:86–93. doi: 10.1016/s0006-291x(87)80090-6. [DOI] [PubMed] [Google Scholar]
- 21.Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Strieter RM, Kunkel SL. Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitis: cross-talk between MCP-1 and leukotriene B4. J Immunol. 1999;163:6148–6154. [PubMed] [Google Scholar]
- 22.Canetti C, Silva JS, Ferreira SH, Cunha FQ. Tumour necrosis factor-alpha and leukotriene B(4) mediate the neutrophil migration in immune inflammation. Br J Pharmacol. 2001;134:1619–1628. doi: 10.1038/sj.bjp.0704403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramos CD, Canetti C, Souto JT, Silva JS, Hogaboam CM, Ferreira SH, Cunha FQ. MIP-1alpha[CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF-alpha and LTB4. J Leukoc Biol. 2005;78:167–177. doi: 10.1189/jlb.0404237. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira SH, Canetti C, Ribeiro RA, Cunha FQ. Neutrophil migration induced by IL-1beta depends upon LTB4 released by macrophages and upon TNF-alpha and IL-1beta released by mast cells. Inflammation. 2008;31:36–46. doi: 10.1007/s10753-007-9047-x. [DOI] [PubMed] [Google Scholar]
- 25.Rocco PR, Zin WA. Pulmonary and extrapulmonary acute respiratory distress syndrome: are they different? Curr Opin Crit Care. 2005;11:10–17. doi: 10.1097/00075198-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Ichinose F, Zapol WM, Sapirstein A, Ullrich R, Tager AM, Coggins K, Jones R, Bloch KD. Attenuation of hypoxic pulmonary vasoconstriction by endotoxemia requires 5-lipoxygenase in mice. Circ Res. 2001;88:832–838. doi: 10.1161/hh0801.089177. [DOI] [PubMed] [Google Scholar]
- 27.Bitto A, Minutoli L, David A, Irrera N, Rinaldi M, Venuti FS, Squadrito F, Altavilla D. Flavocoxid, a dual inhibitor of COX-2 and 5-LOX of natural origin, attenuates the inflammatory response and protects mice from sepsis. Crit Care. 2012;16:R32–R43. doi: 10.1186/1364-8535-16-R32. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Cybulsky MI, McComb DJ, Movat HZ. Neutrophil leukocyte emigration induced by endotoxin: mediator roles of interleukin 1 and tumor necrosis factor alpha 1. J Immunol. 1988;140:3144–3149. [PubMed] [Google Scholar]
- 29.Watanabe K, Konishi K, Fujioka M, Kinoshita S, Nakagawa H. The neutrophil chemoattractant produced by the rat kidney epithelioid cell line NRK-52E is a protein related to the KC/gro protein. J Biol Chem. 1989;264:19559–19563. [PubMed] [Google Scholar]
- 30.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L, Nakahira K, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med. 2012;185:1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Moltke J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, van Rooijen N, Brown CR, Krantz BA, Leppla SH, Gronert K, et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490:107–111. doi: 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson AC, Reddy J, Nazareno R, Sobel RA, Nicholson LB, Kuchroo VK. IL-10 plays an important role in the homeostatic regulation of the autoreactive repertoire in naive mice. J Immunol. 2004;173:828–834. doi: 10.4049/jimmunol.173.2.828. [DOI] [PubMed] [Google Scholar]
- 33.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O’Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 34.Coccia M, Harrison OJ, Schiering C, Asquith MJ, Becher B, Powrie F, Maloy KJ. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med. 2012;209:1595–1609. doi: 10.1084/jem.20111453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Besnard AG, Togbe D, Couillin I, Tan Z, Zheng SG, Erard F, Le Bert M, Quesniaux V, Ryffel B. Inflammasome-IL-1-Th17 response in allergic lung inflammation. J Mol Cell Biol. 2012;4:3–10. doi: 10.1093/jmcb/mjr042. [DOI] [PubMed] [Google Scholar]
- 36.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffin GK, Newton G, Tarrio ML, Bu DX, Maganto-Garcia E, Azcutia V, Alcaide P, Grabie N, Luscinskas FW, Croce KJ, et al. IL-17 and TNF-α sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol. 2012;188:6287–6299. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freitas A, Alves-Filho JC, Victoni T, Secher T, Lemos HP, Sônego F, Cunha FQ, Ryffel B. IL-17 receptor signaling is required to control polymicrobial sepsis. J Immunol. 2009;182:7846–7854. doi: 10.4049/jimmunol.0803039. [DOI] [PubMed] [Google Scholar]
- 39.Arraes SM, Freitas MS, da Silva SV, de Paula Neto HA, Alves-Filho JC, Auxiliadora Martins M, Basile-Filho A, Tavares-Murta BM, Barja-Fidalgo C, Cunha FQ. Impaired neutrophil chemotaxis in sepsis associates with GRK expression and inhibition of actin assembly and tyrosine phosphorylation. Blood. 2006;108:2906–2913. doi: 10.1182/blood-2006-05-024638. [DOI] [PubMed] [Google Scholar]
- 40.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 41.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwarzenberger P, Kolls JK. Interleukin 17: an example for gene therapy as a tool to study cytokine mediated regulation of hematopoiesis. J Cell Biochem Suppl. 2002;38:88–95. doi: 10.1002/jcb.10054. [DOI] [PubMed] [Google Scholar]
- 43.Laan M, Cui ZH, Hoshino H, Lötvall J, Sjöstrand M, Gruenert DC, Skoogh BE, Lindén A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 44.Witowski J, Pawlaczyk K, Breborowicz A, Scheuren A, Kuzlan-Pawlaczyk M, Wisniewska J, Polubinska A, Friess H, Gahl GM, Frei U, et al. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J Immunol. 2000;165:5814–5821. doi: 10.4049/jimmunol.165.10.5814. [DOI] [PubMed] [Google Scholar]
- 45.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]