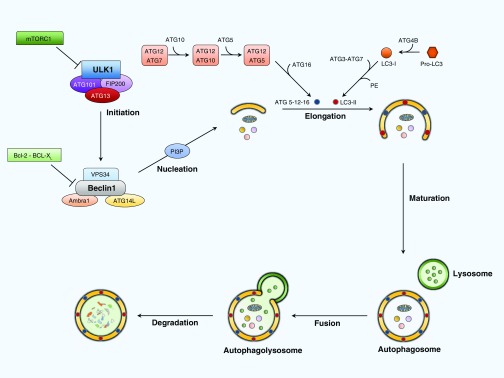
Figure 1.
Molecular mechanism of autophagy. Environmental signals modulate mammalian target of rapamycin (mTOR) complex 1 (mTORC1), negatively regulating autophagy by inhibiting the uncoordinated-51–like kinase 1 (ULK1) complex consisting of ULK1, ATG101, ATG13, and FIP200. Starvation and low ATP levels down-regulate mTOR and directly stimulate the ULK1 complex. The ULK1 complex positively regulates autophagy by activating the Beclin 1 interacting complex, which consists of Beclin 1 (BCL2 family proteins), VPS34 (a class III phosphatidylinositol-3 kinase), and ATG14L. This increases the levels of phosphatidylinositol 3-phosphate (PI3P), which promotes the nucleation of autophagosomal membrane. The elongation of the autophagosome membrane requires two ubiquitin-like conjugation systems. The first is the ATG5–ATG12 complex, which is conjugated by ATG7 and ATG10 enzymes. The second one requires the ubiquitin-like protein microtubule–associated protein 1 light chain 3 (LC3), also called ATG8, which is cleaved by ATG4B into LC3B-I. LC3B-I turns into the active LC3B-II after conjugation with phosphatidylethanolamine by ATG3 and ATG7. Once the double-membrane autophagosome is complete, it fuses with a lysosome to form the autophagolysosome to degrade the autophagosome contents. ATG, autophagy-related protein; FIP200, focal adhesion kinase family interacting protein of 200 kD; VPS34, vacuolar protein sorting 34.