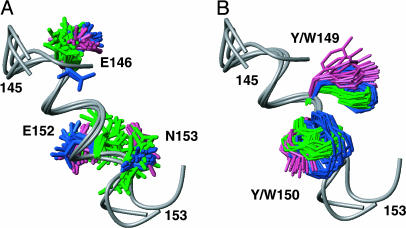
Fig. 5.
Conserved structural motif on the surface of the globular domains of chPrP, tPrP, and hPrP. There is no apparent role in stabilization of the protein fold, indicating that this motif might be conserved for reasons of the protein function. hPrP, chPrP, and tPrP have been superimposed for local best fit of the backbone atoms of residues 146–153, which form the helix α1 (the backbone is shown as a gray spline function). (A) With the side chains of the residues E146, E152, and N153. (B) With the side chains of the residues 149 and 150 (W or Y in the different species; see Fig. 1). hPrP side chains are shown in pink, chPrP side chains are shown in blue, and tPrP side chains are shown in green.