Abstract
Objective: To describe the growth and outcomes of the Palliative Care Research Cooperative Group (PCRC).
Background: Despite advances, significant gaps remain in the evidence base to inform care for people with serious illness. To generate this needed evidence and bolster research capacity, the Palliative Care Research Cooperative (PCRC) group was formed.
Methods: The PCRC supports investigators in the conduct of multisite clinical studies. After developing a governance structure and completing a proof of concept demonstration study, the PCRC expanded its infrastructure to include additional resource cores (Clinical Studies; Measurement; Data Informatics and Statistics; and Caregiver Studies). The PCRC also supports an Investigator Development Center as many palliative care investigators valued opportunities to advance their skills. Additional key aspects of PCRC resources include a Scientific Review Committee, a Publications Committee, and initiatives to purposefully engage investigators in a community of palliative care science.
Results: The PCRC has grown to over 300 members representing more than 130 distinct sites. To date, the PCRC has supported the submission of 51 research applications and has engaged in 27 studies. The PCRC supports investigator research development needs through webinars and clinical trials “intensives.” To foster a sense of community, the PCRC has convened biannual meetings, developed special interest groups, and regularly communicates via a newsletter and its website.
Conclusion: With a particular focus on facilitating conduct of rigorous multisite clinical studies, the PCRC fosters an engaged multidisciplinary research community, filling an important void in generating and disseminating evidence that informs the provision of high-quality care to people with serious illness.
Keywords: : investigator development, multi-site studies, palliative care, palliative care research, research
Background
The growth of people living with serious illness has not been accompanied by the same advancement in evidence for how to care for them. Evidence growth has been impeded by small, single-site trials, unrepresentative study cohorts, and studies lacking sufficient rigor.
Other fields have advanced their evidence through large, diverse, multisite trials.1,2 To conduct these trials, these fields, namely cardiology and oncology, have devised an infrastructure to support large studies.3,4 Palliative care has had neither the infrastructure nor scientific workforce to answer many important questions relevant to improving care for those with serious illness and their caregivers.
The National Institute for Nursing Research (NINR) funded the Palliative Care Research Cooperative (PCRC) to address this need. Formed in 2010, the PCRC provides a framework to support investigators as they conduct high-quality multisite palliative care research.5 The PCRC offers core resources in caregiver research, measurement, clinical study design, and statistical analyses and provides investigators with opportunities to enhance their research skills. The PCRC is unique in its engagement of multidisciplinary investigators, its focus on multiple diseases and conditions, and its access to clinical populations across the care continuum. In this article, we describe the growth and evolution of the PCRC, following up on the initial description published in this journal in 2010. We also highlight accomplishments, lessons learned, and plans for the future.
Methods
Phase 1: building the cooperative and conducting the first proof of concept study
Prior articles describe the founding of the PCRC.5–10 The PCRC was formed in 2010 to fill an important void in palliative care research. The PCRCs overarching mission is to support the generation and application of meaningful evidence relevant to care of patients with advanced or potentially life-limiting illnesses and their caregivers. Specific objectives include expanding research capacity, conducting high-impact clinical trials, and training new investigators. Phase 1 of PCRC development, which was supported by the NINR UC4 funding (UC4NR12584), entailed building the cooperative and conducting a proof of concept multisite randomized trial. Phase 1 focused on implementing the necessary steps to launch the PCRC.5 Key components of these initial years (2011–2013) included the following.
• identification of initial members and sites;
• creation and ratification of the PCRC charter, standard operating procedures, and terms of reference;
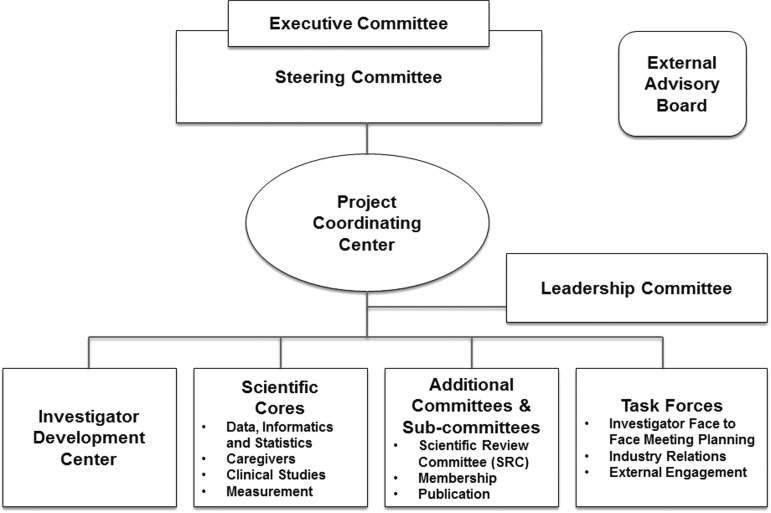
• development of a governance structure (Fig. 1);
• creation of the initial PCRC core resources; and
• conduct of the initial proof of concept multisite randomized clinical trial.
FIG. 1.
PCRC organizational structure. PCRC, Palliative Care Research Cooperative.
Infrastructure developed under the initial grant includes two principal organizational components, the Project Coordinating Center (PCC) and the Data, Informatics, and Statistics Core that remain in place today.
Phase 2: expansion and growth
NINR U24 funding (1U24NR014637; 2013–2018) has supported Phase 2. Initial NINR funding resulted in a well-functioning national cooperative group dedicated to palliative care and end-of-life (PCEOL) research. The U24 funded the augmentation of the PCRC to offer more powerful resources for collaborative PCEOL research. The overarching objective of Phase 2 has been to amplify the role of the PCRC as a national resource for high-quality, collaborative, multisite, PCEOL research. In Phase 2, we added an Investigator Development Center (IDC) and three research cores (Caregiver, Clinical Studies, and Measurement), as well as a Steering Committee, External Advisory Board, and key committees (Scientific Review, Membership, Publications) to enhance methods of PCEOL research, develop the PCEOL research workforce, and enhance the reach of the PCRC. Initially, the PCRC had a two-member executive team. As the PCRC grew, the Steering Committee recommended expanding the executive team, both for running the PCRC and also to insure a succession plan. Thus, a three-person executive team was created to include the Co-Chairs and the Director of the Scientific Review Committee. The PCRC currently has three Co-Chairs, all multiple PIs of the U24 (J.K., C.R., K.P.).
PCRC Cores and Centers
PCRC Cores and Centers (Fig. 1 and Table 1) are intentionally interfacing and coordinated, providing a matrixed environment of support for palliative care investigators. The Cores and Centers serve to improve the rigor of palliative care research, increase the competitiveness of research applications, and enhance study efficiency. Cores and Centers provide consultation to investigators. Use of Core resources is available (supported by the U24) free of charge as investigators prepare their grant applications. Investigators can budget to consult with Core resources postaward.
Table 1.
Palliative Care Research Cooperative Cores and Centers
| Core name | Core lead(s) | Purpose | Resources provided |
|---|---|---|---|
| Caregiver Research | Betty Ferrell, PhD, RN | Facilitates advancement of caregiver research, elevating quality and prominence of caregiver research, and integrating caregiver concerns into studies. | Consulting: study design, measurement |
| Elaine Wittenberg, PhD | Library of preferred caregiver study designs, measures, and instruments | ||
| Data, Informatics, and Statistics Core | Katie Colborn, PhD Diane Fairclough, DrPH Greg Samsa, PhD |
Provides expertise in statistical and data-related aspects of multisite study design, evaluation, quality assurance, data analysis and reporting, data sharing, and clinical informatics. | Consulting: analytic strategies for multisite studies |
| Deidentified data repository | |||
| Common data elements | |||
| Data quality assurance | |||
| Clinical Studies | Frank Keefe, PhD | Advises investigators regarding appropriate interventions and clinical trials study design, providing guidance materials and methods that are standardized, validated, and relevant to PCEOL interventions. | Consulting: study design, biobehavioral intervention development |
| Tammy Sommers, PhD | Library of study protocols | ||
| Measurement | Antonia Bennett, PhD | Provides expertise in selection and application of study measures relevant to PCEOL studies. | Consulting: outcome and measure selection |
| Measurement library | |||
| Investigator Development Center (IDC) | Christine Ritchie, MD, MSPH | Strengthens and enhances PCEOL investigator pipeline and expertise, especially in conduct of multisite studies | Webinars |
| Clinical trial intensives | |||
| Pilot grant awards | |||
| Mentor matching | |||
| Project Coordinating Center (PCC) | Jean Kutner, MD, MSPH | Supports PCRC investigators and sites in logistical and technical aspects of study development, management, and conduct. | Communication |
| Site and investigator support | |||
| Protocol development | |||
| Site training | |||
| Regulatory coordination | |||
| Financial/budget management |
PCEOL, palliative care and end-of-life; PCRC, Palliative Care Research Cooperative.
The PCRC Cores and the PCC offer “on-ramps” to investigators, providing support to effectively conduct multisite studies. For example, the Clinical Studies and Data, Informatics, and Statistics Cores may serve as starting points for investigators who are thinking through study design and appropriate analyses. These Cores offer both one-on-one consultation and study guidance through representation on the Scientific Review Committee. As studies become more clearly conceptualized, investigators may then draw on the expertise and tools available through the Measurement and Caregiver Cores, both of which offer an array of validated participant and caregiver assessment tools relevant to palliative care populations. As a study moves from concept to grant submission, and ultimate conduct, the PCC provides ongoing practical assistance on budgets, timelines, and processes, along with site identification, protocol guidance, site training, and site audits.
PCRC committees
PCRC committees serve key functions in achieving the overarching goals of the PCRC. The Scientific Review Committee aims to improve research quality and rigor by requiring that investigators who are collaborating with the PCRC submit a detailed letter of intent (http://palliativecareresearch.org/research/investigators/extramural/), which includes information on study purpose, significance, investigators, innovation, approach, and how investigators plan to collaborate with the PCRC. The letter of intent also provides information about common data elements and the planned outcome measurement instruments. Two members of the Scientific Review Committee and a PCRC statistician provide recommendations for strengthening the science. This process informs the PCRC letter of support that is intended to help grant reviewers understand the role of the PCRC and bolster their confidence in the investigator's ability to achieve the study aims. Once a study is funded, the Scientific Review Committee reviews the study protocol.
The PCRC Publications Committee encourages dissemination of new knowledge by working with investigators to assure that PCRC-supported research follows PCRC Authorship Guidelines and is published in a timely manner. The PCRC Membership Committee reviews new member applicants and determines their level of participation (full, junior, or affiliate), assures that members continue to meet membership requirements, and guides junior members to advance to full membership (http://palliativecareresearch.org/members/interested-in-pcrc-membership).
Investigator engagement
The PCRC engages its growing membership via a multimodal approach. First, the PCRC meets face-to-face twice yearly in investigator meetings. In these meetings, we update members on PCRC progress, provide opportunities for both informal and structured professional networking, and include didactic scientific and career development presentations. Investigators funded through the PCRC describe their work along with how they interface with the PCRC. In between face-to-face meetings, the PCRC provides quarterly newsletters to update members on newly funded projects, findings from studies, and funding opportunities. The PCRC website (palliativecareresearch.org) serves as a central and continuously updated resource. The PCRC was built on a commitment to continuous improvement and functioning as a learning organization. Thus, the PCRC regularly seeks feedback (e.g., after collaborating on grant application submissions with investigators, face-to-face meetings, webinars, and intensives). The PCRC has also developed mechanisms for continuous monitoring and improvement of its operations to make the organization efficient and effective.
Investigator development
Even though many members of the PCRC are experienced investigators, many are new to palliative care research and most are new to multisite clinical trials. The IDC was designed to address this gap. The PCRC IDC supports member skill development through webinars, pilot studies, and intensives, in addition to didactic sessions at investigator meetings. We post monthly webinars to the PCRC website. The pilot study program provides opportunities for investigators to obtain preliminary data needed for future extramurally funded research activities through the PCRC. In addition, the awards offer opportunities for investigators to participate in investigator development activities and to be mentored by senior investigators. To facilitate junior investigator experience with grant reviews and to optimize an interdisciplinary approach to the review process, each pilot study application has three reviewers: a senior researcher, a junior researcher, and a nurse researcher. Pilot awardees benefit from the perspectives of three reviewers; reviewers benefit from each other's research expertise, and junior members learn how to conduct reviews. There are unique and particularly challenging aspects to palliative care clinical trials. The clinical trial intensives, modeled after the successful clinical trial intensives developed at the National Heart, Lung, and Blood Institute, immerse selected investigators into a team-based grant writing experience that gives them the opportunity to walk through grant planning and writing alongside experienced palliative care research faculty and statisticians.
Results
The PCRC has achieved its initial goals through collaboration with NINR, development of new research infrastructure and resources, creation and continuous refinement of processes, and engagement of a growing collaborative interdisciplinary research community. Table 2 summarizes key contributions and achievements.
Table 2.
Palliative Care Research Cooperative Key Contributions and Achievements
| Structural components | Processes | Outcomes (January 2011–2017) |
|---|---|---|
| Centers | ||
| Project Coordinating Center | Project support • Budget development • Site selection support • Letter of intent template • Protocol template with required data sharing language • Protocol instructions for investigators • Institutional Review Board (IRB) suggested language • Study site onboarding and training • Study site monitoring |
70 letters of support 51 grant applications supported 11 extramurally funded grants (e.g., NIH, ACS) supported |
| Communication • Quarterly newsletters • Monthly e-mail updates • PCRC website (http://palliativecareresearch.org) • Social media (Facebook, Twitter) |
||
| IDC | • Monthly webinars • Mentor/mentee matching • Advisory role for content of face-to-face investigator meetings • Palliative care clinical trial intensives • Collaboration with the National Palliative Care Research Center (NPCRC) |
• 17 pilot grants funded (9 more to be funded 7/2017) • Establishment of special interest groups • 2 palliative care clinical trial intensives attended by 26 investigators • Monthly webinars, attended by an average of 45 participants; archived on PCRC website (http://palliativecareresearch.org/corescenters/idc/idc-webinars) • 4 study recruitment training videos (://palliativecareresearch.org/resources/videos/) |
| Cores | ||
| Data, Informatics, and Statistics Core | Consulting: • Statistical • Electronic data capture • Database development • Data quality assurance • Data repository curation |
• Common data elements • Data repository of deidentified data available for secondary analyses • Consulting with 52 investigators |
| Caregiver Core | Caregiver research expertise | • Caregiver measurement library • Consulting with 59 investigators |
| Measurement Core | Measurement expertise | • Measurement library • Consulting with 31 investigators |
| Clinical Studies Core | Clinical studies design expertise | • Consulting with 11 investigators • Behavioral intervention protocol library |
| Committees | ||
| Executive | Primary oversight of PCRC strategy, finances, and operations | |
| Steering | Advises executive committee on strategic directions | Review and approval of: Membership, Scientific Review Committee recommendations |
| Membership | Monitors compliance with PCRC membership guidelines | Membership growth (see figures) |
| Reviews applications for PCRC membership and makes membership recommendations to PCRC Executive and Steering Committees | ||
| Scientific Review | Reviews proposals for new PCRC-supported research to determine potential impact, feasibility, and appropriateness for conduct within the PCRC with a goal of strengthening the science of palliative care and end-of-life research | 42 LOIs reviewed |
| 11 protocols reviewed | ||
| Publication | Facilitates, encourages, and coordinates dissemination of results from PCRC-supported studies, and knowledge derived therefrom | 11 publications directly attributable to UC4 and U24 funding. |
| Additional 45 publications supported by PCRC activities. | ||
| External Advisory Board | Provides guidance to the PCRC Steering and Executive Committees | Serves as the PCRCs external horizon scanning function, providing advice on overall trends, threats, and opportunities to inform PCRC strategic planning. Twice yearly meetings. |
LOI, letter of intent.
PCRC growth
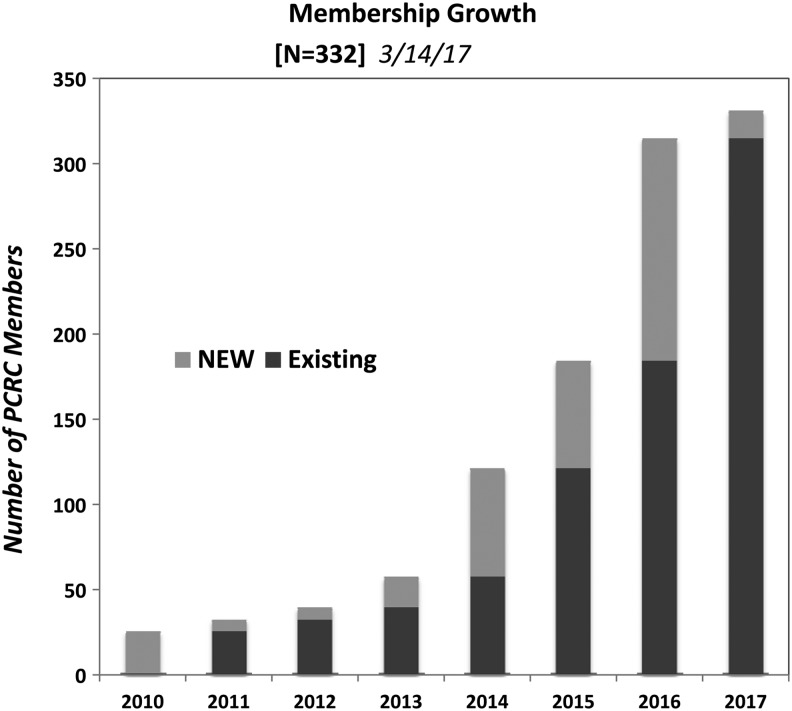
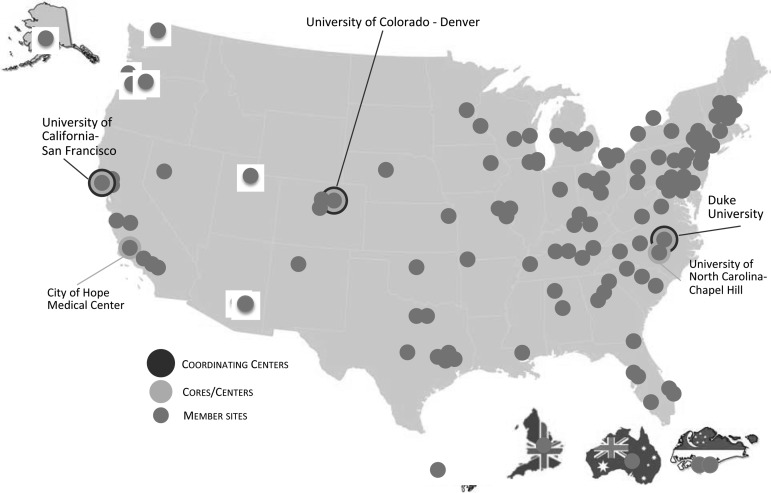
As noted in Figures 2 and 3, the PCRC has seen tremendous growth since inception, increasing from initial membership of 24 individuals and 12 sites to a current membership of 332 individuals and 134 geographically dispersed sites. Among current members, 43% are full members, 48% are junior members, and 9% are affiliate members.
FIG. 2.
PCRC growth.
FIG. 3.
PCRC member sites.
Investigator development and community building
The pilot award program has received 38 applications and selected 17 pilot awards for funding. More than 100 reviewers have participated in pilot grant reviews. To date, 26 investigators have participated in PCRC clinical trial intensives. Evaluations have been uniformly positive; 20 grant proposals were subsequently submitted by intensive participants. Of 21 respondents to a six-month follow-up survey, 100% indicated that the intensive had had a positive impact on their career.
Partnership and engagement with the NINR
In keeping with the spirit of the structure of the U24 cooperative agreement, the partnership between PCRC leadership and the NINR has been key to the PCRC development and expansion as a national leader in palliative care science. The initial UC4 funding mechanism and the current U24 explicitly require active engagement between the funder and the PCRC principal investigators. This collaboration and engagement assure a commonality of purpose and relevance to national PCEOL research priorities and enhance the visibility, legitimacy, and accessibility of this resource to investigators and other funding agencies. Examples of the close partnership include jointly agreed upon milestones and collaboration on selection of pilot awards as well as pre- and postaward communication with investigators. In addition, the collaborative interactions between the NINR program and scientific staff and the PCRC leadership position the PCRC to be responsive to new and future clinical trial initiatives and new NIH policies and programs. Maintaining both a collaborative and strategic partnership between the NINR Office of End-of-Life and Palliative Care Research and the PCRC facilitates the ongoing and forward-thinking strategies within the U24 funding mechanism that allows the PCRC to continue to grow. An example is the evolution of the PCRC IDC to offer innovative training programs in palliative science to promote a sustainable and diverse research workforce.11
The PCRC Centers and Cores are aligned with NIH emphasis on new model programs that will scale-up the sustainability of evidence-based interventions and create new tools and research methods that will enable interventions that are effectively delivered with communities of practice.12 In keeping with a new emphasis by the NIH on dissemination and implementation research in health,13,14 the PCRC Centers and Cores create novel resources and new opportunities for researchers to participate in integrated, team science and, through their members and sites, the potential for earlier uptake of research findings into research and clinical care. The PCRCs development of a deidentified data repository also aligns with the efforts of the NIH to create resources to ensure data are shared broadly, consistently, safely, and efficiently for appropriate use across the research community.15 The capability of the PCRC to ensure that deidentified datasets are maintained, queried, and reconfigured will facilitate new analyses and contribute to a cost-effective and efficient means for advancing PCEOL research. In addition, the creation of a seminal central repository of palliative research data will serve to facilitate standardization of how data are reported and permit new studies to build on the data collected to enhance the value of each study.16–18 The capability of the PCRC to acquire and retain a nationally representative research database supports NIH initiatives such as the Big Data to Knowledge (BD2K) initiative aimed at facilitating discovery, supporting new knowledge, and maximizing community engagement.19 The potential of the PCRC to train researchers in palliative care science on big data methodology, statistical design, and incorporation of informatics into multitrial designs will allow researchers to maximize the potential of existing data and enable new directions for palliative research.19
The PCRC has incorporated into its studies the value of patient-centered data. Through the resources of the Measurement and Caregiver Cores, many PCRC-supported studies include trial designs that incorporate individuals and family caregivers. This emphasis is in keeping with many of the efforts of the NIH precision medicine initiative (PMI), which includes the patients' perspective in research and facilitates their maximum engagement in their care.20,21 Similar to PMI approaches, PCRC research is guided by inclusion of individualized and/or family-centered trials that seek to find better prevention strategies, treatment selections, and new and novel therapies.20,21 The PCRC and its Centers and Cores have recognized the centrality of patient engagement in clinical trials and their role in healthcare decision-making in palliative care research to facilitate best outcomes.22
The NIH has also undergone a multifaceted effort to improve the quality and efficiency of clinical trials to ensure rigor and efficiency of research within the clinical trial setting.23 The PCRC was developed as a national resource in the conduct of evidence-based clinical trial research and to build a cadre of investigators who maintain scientific rigor and oversight. In keeping with recent NIH changes to address efficiency, accountability, and transparency of clinical research,23 the PCRC has emerged as a national resource to ensure advancement of palliative knowledge and improve palliative care. The PCRC continues as a national resource to assist researchers with complex trial designs, accrual of sample sizes that are representative of national populations, and to provide the expertise and resources needed to ensure data from these trials are shared broadly and efficiently. As the NIH enacts new policies for training of investigators and staff responsible for clinical trial oversight, use of centralized IRBs, data safety monitoring requirements, and enhancing the design, conduct, and oversight of trials, the PCRC Cores and responsible data sharing infrastructure will continue to ensure transparency of NIH-supported multisite clinical trials.23
Discussion
The PCRC has succeeded in creating a research infrastructure that addresses key needs in the field. Particular strengths of the PCRC include an engaged and growing interdisciplinary research community, diverse membership that includes research sites in varied geographic regions, both academic- and community-based sites and methodologic cores specific to research challenges and priorities relevant to the palliative care and end-of life-population. Because the PCRC promotes the use of common data elements and requires that studies conducted through the PCRC maintain their deidentified data in a common repository, the PCRC also serves as an avenue for investigators to engage in secondary data analysis and comparative effectiveness research in a way currently not available to palliative care researchers through other means.
A key facilitator of PCRC successes is a highly collaborative and engaged investigator community. PCRC members generously volunteer their time, actively serve on committees, review pilot grant applications, and present at PCRC investigator meetings. Despite it being a new venture, investigators have been willing to take a risk on the PCRC, submitting their extramural grant applications, working with the PCRC through process improvements, and supporting the science and the work of colleagues by serving as study sites. The PCRC has been fortunate to have the active engagement of senior investigators in the field, who have been generous with their time and expertise, and who have endorsed the PCRC as a key resource for advancing and enhancing palliative care research.
The shared leadership model among the PCRC executive team, with open communication and truly shared governance, has facilitated efficiency and modeled a team science approach, which is critical to successful palliative care research. The PCRCs highly competent operations staff autonomously handle day-to-day operations, allowing the executive committee to focus on more strategic and oversight responsibilities.
Collaboration with and support by the NINR, along with advocacy of and proactive communication by and accessibility to the PCRC of NINR Scientific and Program Officers, have been key to the PCRC success. Partnership with the National Palliative Care Research Center (NPCRC), creating purposeful synergies, particularly as related to investigator development, has enriched and unified the palliative care research community. Finally, the PCRC owes some of its success to institutional support from Duke, the University of Colorado, and the University of California, San Francisco, demonstrating flexibility and a willingness to work with PCRC leadership to facilitate virtual, cross-institutional structures and processes.
While the PCRC has successfully achieved its initial goals, there remain significant challenges and opportunities. The PCRC business model, as proposed in the 2013 U24 award, was premised on gradually increasing reliance on extramurally funded multisite studies to support PCRC infrastructure. As the PCRC began to collaboratively work with investigators to support their multisite studies, it became clear that, given required sample size needs in often heterogeneous palliative care populations, these studies required budgets that regularly exceed budget caps for most funding agencies. The PCRC leadership realized early on in the U24 funding that it is not possible to support cooperative group infrastructure on R-type grants alone. The PCRC has thus relied on rebudgeting within its original U24-awarded amount to support fundamental infrastructure components (e.g., Cores, Centers) that have been identified as essential resources by investigators. As we look to the future of the PCRC, we are exploring funding and business models that best accommodate the realities of the required sample sizes and budgets for conducting rigorous multisite studies.
Another significant challenge and opportunity involves tremendous investigator development needs. It has become apparent that there are significant investigator knowledge gaps, particularly related to designing, conducting, and analyzing multisite clinical studies. In recognition of these greater than anticipated investigator development needs, the originally proposed “Junior Investigator Development Core” was renamed as the “Investigator Development Core.” In addition, the PCRC has identified a significant lack of local research resources at many members' home institutions. Of particular note is lack of access to statistical resources and inexperienced or minimal pre- and postaward research administration resources. While the PCRC can fill some local institutional gaps, it does not have the resources, or capability, of making up for all local deficits.
The PCRC has tracked easily quantifiable outcomes (e.g., number of consultations provided by Cores, number of pilot grants awarded, number of extramurally funded grants supported, Table 2). Nevertheless, we have found it more challenging to effectively track the impact of the PCRC and its growing membership in quantifiable ways. The PCRC has identified as a key priority for the future developing an efficient approach to identifying meaningful outcomes and building systems to track and report these metrics.
While the executive team has been fortunate to work within, and across, academic institutions that have supported the mission of the PCRC, the PCRC and its investigators have encountered significant issues stemming from existing traditional research silos and structures that complicate multisite, cooperative group-based research. For example, a number of investigators with whom the PCRC has collaborated have encountered roadblocks related to allocation of indirects, IRB review and approval for multisite research, and variable institutional rules related to subcontracting and payment of study sites. As studies begin the process of providing deidentified data to the PCRC data repository, issues of data ownership also must be addressed. The future success of the PCRC likely will include use of a central IRB to provide support to its members and increase the speed with which we can do our work.
Finally, while the PCRC has been generously funded by its existing U24 cooperative agreement, the success of the PCRC is fundamentally reliant on significant volunteer and unfunded effort from its Core directors, and committee and task force chairs and members. The majority of attendees at PCRC investigator meetings support attendance at these meetings out of their own resources. In the increasingly tight funding environment, with multiple competing demands, heavy reliance on goodwill and volunteerism is a potential organizational weakness.
Conclusion
The PCRC is uniquely contributing to advancing the science of palliative and end-of-life care through creation of a multidisciplinary research community as well as infrastructure and resources that enhance the rigor of the science. The PCRC, unlike other cooperative groups, solely focuses on palliative care research. It has purposely been designed as a learning organization, constantly evaluating and revising its procedures to assure relevance in a rapidly changing external context. As the PCRC looks to the future, it expects to expand it resources to meet the evolving research priorities, facilitating conduct of studies that address the more complicated and complex questions relevant to care of people with advanced or potentially life-limiting illness, ultimately improving care for this vulnerable population.
Acknowledgments
The authors are deeply grateful to the PCRC operations team, without whom the work of the PCRC would not have been possible: Molly Gavigan, Rachael Kendrick, Carey Candrian, Jordan Lodato, Sarah Garrigues, Sherry Andrews, Sangeetha Tadimalla, Nicole Thompson, and Gloria Bass. The authors also acknowledge the contributions of its members, particularly those who have assumed leadership roles within the PCRC, those who have collaborated with the PCRC on grant applications and on studies, and those who have volunteered their time as reviewers and as committee and task force members. The authors are also tremendously grateful to the External Advisory Board members for sharing their wisdom and perspective. Finally, the authors acknowledge the vision of its founding principal investigator, Amy Abernethy, without whom the PCRC would not have come into being.
This work is funded by National Institute of Nursing/National Institutes of Health (UC4-NR012584, U24-NR014637).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Institute of Medicine (US) Forum on Drug Discovery, Development, and Translation: Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary. Washington (DC): National Academies Press (US); 2010. 3, Challenges in Clinical Research. www.ncbi.nlm.nih.gov/books/NBK50888 (last accessed April18, 2017) [PubMed] [Google Scholar]
- 2.Concannon TW, Guise J-M, Dolor RJ, et al. : A national strategy to develop Pragmatic Clinical Trials Infrastructure. Clin Transl Sci 2014;7:164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salahudeen AA, Patel MI, Baas P, et al. : Overview of thoracic oncology trials in cooperative groups around the globe. Clin Lung Cancer 2017;18:5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson RM, McNeer JF, Logan L, et al. : A cooperative network of trained sites for the conduct of a complex clinical trial: A new concept in multicenter clinical research. Am Heart J 2006;151:451–456 [DOI] [PubMed] [Google Scholar]
- 5.Abernethy AP, Aziz NM, Basch E, et al. : A strategy to advance the evidence base in Palliative Medicine: Formation of a palliative care research cooperative group. J Palliat Med 2010;13:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lodato JE, Aziz N, Bennett RE, et al. : Achieving palliative care research efficiency through defining and benchmarking performance metrics. Curr Opin Support Palliat Care 2012;6:533–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leblanc TW, Abernethy AP, Currow DC, Kutner JS: Considerations in reporting palliative care clinical trials: Standardizing information reported and authorship practices. Curr Opin Support Palliat Care 2012;6:494–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritchie CS, Currow DC, Abernethy AP, Kutner JS: Multisite studies offer a solution to recruitment challenges in palliative care studies. J Palliat Med 2013;16:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanson LC, Bull J, Wessell K, et al. : Strategies to support recruitment of patients with life-limiting illness for research: The palliative care research cooperative group. J Pain Symptom Manage 2014;48:1021–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutner JS, Blatchford PJ, Taylor DH Jr., et al. : Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: A randomized clinical trial. JAMA Intern Med 2015;175:691–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.https://biomedicalresearchworkforce.nih.gov/index.htm (Last accessed March16, 2017)
- 12.Tabak RG, Padek MM, Kerner JF, et al. : Dissemination and implementation science training needs: Insights from practitioners and researchers. Am J Prev Med 2017;52:S322–S329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riley WT: Behavioral and Social Sciences at the National Institutes of Health: Adoption of research findings in health research and practice as a scientific priority. Transl Behav Med 2017; [Epub ahead of print]. DOI: 10.1007/s13142-017-0474-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.https://obssr.od.nih.gov/scientific-initiatives/dissemination-and-implementation (Last accessed February28, 2017)
- 15.www.healthdata.gov (Last accessed February28, 2017)
- 16.https://grants.nih.gov/policy/sharing.htm (Last accessed February28, 2017)
- 17.https://datascience.nih.gov/Blog_HealthData.gov (Last accessed February28, 2017
- 18.Office of Extramural Research: Data Repositories–A Resource Guide. https://grants.nih.gov/grants/data_repositores_samples.doc (Last accessed February21, 2017)
- 19.https://datascience.nih.gov/bd2k (Last accessed March16, 2017)
- 20.www.nih.gov/research-training/allofus-research-program (Last accessed March16, 2017)
- 21.Precision Medicine Initiative (PMI) Working Group Report to the Advisory Committee to the Director, NIH. The Precision Medicine Initiative Cohort Program–Building a Research Foundation for 21st Century Medicine September17, 2015 www.nih.gov/sites/default/files/research-training/initiatives/pmi/pmi-working-group-report-20150917-2.pdf (Last accessed March18, 2017) [Google Scholar]
- 22.Snyderman R, Meade C, Drake C: Value of personalized medicine. JAMA 2016;315:613. [DOI] [PubMed] [Google Scholar]
- 23.Hudson KL, Lauer MS, Collins FS: Toward a new era of trust and transparency in clinical trials. JAMA 2016;316:1353–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]