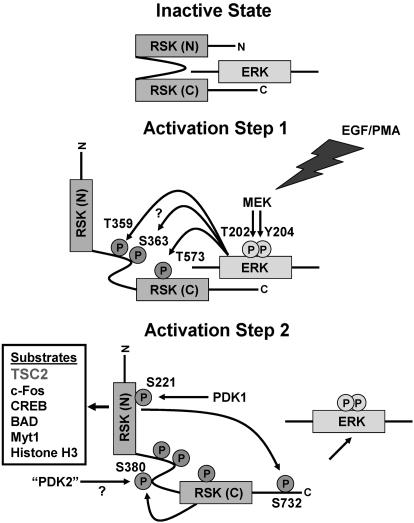
Fig. 4.
Ordered phosphorylation events occur in two main activation steps to fully activate RSK1. In its inactive state, RSK1 is in a complex with inactive ERK1 or ERK2. In activation step one, a variety of extracellular stimuli activate ERK1 and ERK2. Active ERK1 and ERK2 phosphorylate Thr-573 in the activation loop of the carboxyl-terminal kinase domain of RSK1, which leads to the phosphorylation of two sites in its linker region by ERKs or other kinases. Phosphorylation in the linker region is thought to unfold the kinase, priming it for the final activation step. In activation step two, the carboxyl-terminal kinase domain (and/or a putative 3-phosphoinositide-dependent kinase, “PDK2”) phosphorylates Ser-380 and thereby increases the docking of 3-phosphoinositide-dependent kinase-1 (PDK1), which phosphorylates Ser-221 in the activation loop of the amino-terminal kinase domain of RSK1. The final event is autophosphorylation of the carboxyl-terminus by the amino-terminal kinase domain. This event releases ERK, and fully active RSK1 finds a growing number of known substrates located throughout the cell. MEK, mitogen-activated protein kinase kinase; CREB, cAMP response element binding protein.