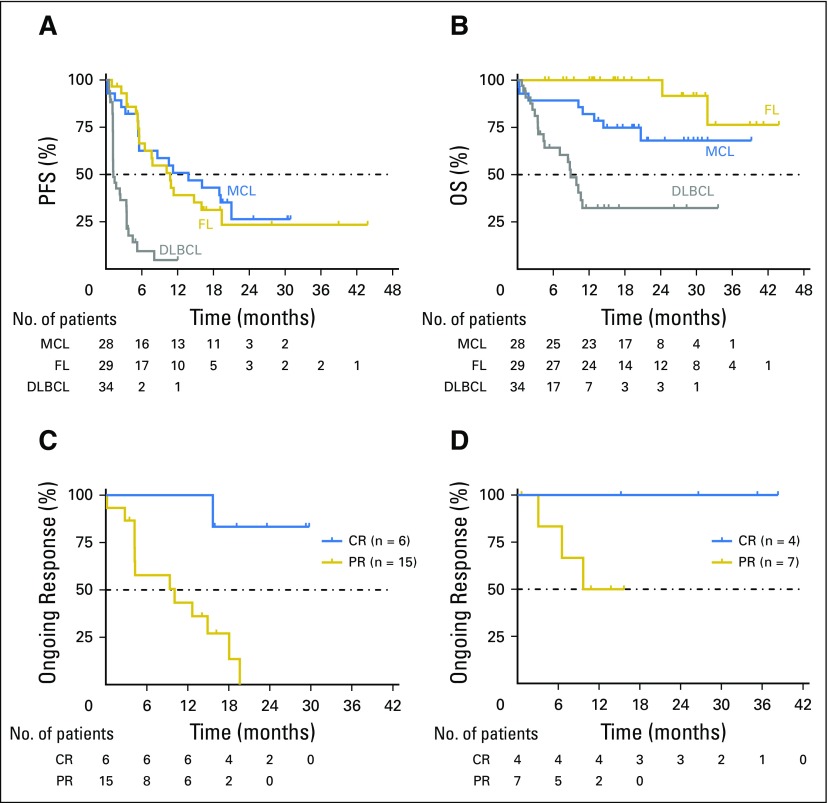
Fig 2.
Durability of antitumor activity of venetoclax. Dashed lines indicate the 50% mark in each panel. (A) Progression-free survival (PFS) of patients with mantle cell lymphoma (MCL), follicular lymphoma (FL), and diffuse large B-cell lymphoma (DLBCL). Of note, three patients with previously chemotherapy-refractory disease, including DLBCL, primary mediastinal large B-cell lymphoma, and MCL, who proceeded to allogeneic stem-cell transplantation after achieving remission with venetoclax were censored when they left the study but remained disease free during the protocol-defined 2-year follow-up period after venetoclax therapy. (B) Overall survival (OS) of patients with MCL, FL, and DLBCL. Duration of response in (C) evaluable patients with MCL and (D) patients with FL who achieved a complete (CR) or partial response (PR).