Table 1.
Drug summary box.
| Name | AZD9291, osimertinib, Tagrisso™ |
|---|---|
| Chemical structure |
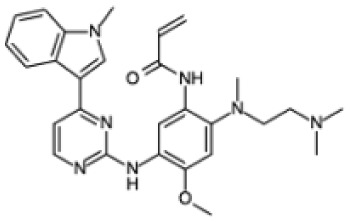
|
| Biochemical name | N-[2-[2-(dimethylamino)ethyl-methylamino]-4-methoxy-5-[[4-(1-methylindol-3-yl)pyrimidin-2- yl]amino]phenyl]prop-2-enamide |
| Pharmacodynamics | Irreversible, covalent bond and inhibition of sensitizing mutation (exon 19 deletion, L858R) and double mutants harboring T790M |
| Pharmacokinetics | Median time to Cmax = 6 h Mean volume of distribution Vss = 986 l Oral clearance = 14.2 l/h Mean half-life = 38 h Time to steady state: 22 days Elimination: 68% fecal and 14% urinary |
| Drug interaction | CYP3A inhibitors or inducers, substrates of CYP3A, BCRP and CYP1A2 |
| Adverse events | Any grade ⩾5%: diarrhea, rash, dry skin, nail toxicity, decreased appetite Select toxicities of special interest: ILD/pneumonitis, QTc interval prolongation, cardiomyopathy, hyperglycemia |
ILD, interstitial lung disease
