Abstract
Soft tissue sarcomas are a rare and multifaceted group of solid tumours. Neoadjuvant chemotherapy is increasingly used to limit loss of function after wide surgical excision with the ultimate aim of improving patient survival. Recently, advances in the identification of effective treatment strategies and improvements in patient risk stratification have been reached. A randomized trial demonstrated that neoadjuvant epirubicin and ifosfamide improves survival of patients affected by five high-risk soft tissue sarcoma histologies of trunk and extremities, including undifferentiated pleomorphic sarcoma, myxoid liposarcoma, synovial sarcoma, malignant peripheral nerve sheath tumours, and leiomyosarcoma. Selection of patients for these treatments is expected to be improved by the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system, as it tailors T-stage categories on primary tumour site and considers a prognostic nomogram for retroperitoneal sarcoma, which also includes soft tissue sarcoma histology and other patient and tumour features not directly included in the TNM staging. Within this framework, this article will present neoadjuvant treatment strategies for high-risk soft tissue sarcoma, emphasizing the most recent advances and discussing the need for further research to improve the effectiveness of neoadjuvant treatments.
Keywords: chemotherapy, neoadjuvant, radiotherapy, soft tissue sarcoma, trial
Background
Soft tissue sarcomas (STS) are a group of rare tumours, accounting for more than 50 different subtypes, which can differ significantly in their disease presentation, response to currently available treatments, and risk of tumour progression.1,2 STS account for about 1% of all solid tumours, with five to six new cases every 100,000 people yearly.3,4 Sarcomas may develop at any age and in virtually all anatomic sites, making them a challenge for medical and surgical oncologists.5
Despite the complexity of these tumours, few treatment options are available.6 Surgery is the standard treatment for primary STS and is aimed at reaching negative tumour excision margins.7,8 Radiotherapy is considered before or after surgery to lower the risk of local recurrence in tumours with worrisome features, such as large size or high grade histology. In some cases radiotherapy may be used to reduce the extent of surgery.9
Several efforts have been put in place over time to improve the quality of surgery, optimize radiotherapy schedules, and refine selection of patients for systemic perioperative treatments, leading to an improvement of patient survival.10,11
Adjuvant systemic therapies have been tested to reduce the risk of metastatic spread after surgery with or without radiotherapy in several randomized controlled trials (RCTs).12 Anthracycline-based regimens using doxorubicin as the main chemotherapeutic agent were used in early studies, while more recent trials have tested anthracycline combined with ifosfamide.13 These treatment strategies offer a survival benefit to patients ranging between 5% and 10%, which has been considered unsatisfactory particularly when balanced against high-grade toxicity.13
Neoadjuvant chemotherapy is increasingly used for patients with locally advanced and high-risk primary sarcomas14,15 and has several advantages over adjuvant systemic treatments. First, when administered preoperatively chemotherapy can improve the chances of performing conservative surgery, resulting in sparing nerves, vessels, and muscle groups with the ultimate aim of reducing the need for amputation and preserving muscle function, especially for extremity STS. In the retroperitoneum, neoadjuvant therapy can reduce the need for extensive multivisceral resection. Another potential benefit of neoadjuvant chemotherapy is an improvement in achievement of negative histologic margins, which are associated with a reduced risk of local recurrence and, to a lesser extent, better survival. Remarkably, when neoadjuvant chemotherapy is delivered with radiotherapy these aims can also be reached indirectly, as chemotherapy acts as a radiosensitizer. Importantly, neoadjuvant chemotherapy can potentially improve patient survival directly through eradication of micrometastatic disease. In this regard, patients can experience significant postoperative complications and delays in starting adjuvant systemic chemotherapy are common for patients with sarcomas. Administering systemic treatments preoperatively can overcome this issue. Finally, pathological response to preoperative chemotherapy can inform decisions about future therapeutic strategies.
Despite these theoretical advantages, the use of neoadjuvant chemotherapy for patients with sarcomas is limited by several issues, such as the well known heterogeneity of these tumours, patients who are older and have comorbidities, and challenges in identifying high-grade tumours at preoperative core biopsy.16 Also a limited number of drugs, mainly cytotoxic agents, are available for patients with early-stage STS.17
This review will present the latest evidence and clinical implications for neoadjuvant chemotherapy in high-risk STS, emphasizing the importance of improving patient risk stratification for identifying those who are likely to benefit from available therapies through new prognostic tools, such as the AJCC TNM staging system and nomograms. Also, this review will illustrate the limitations of administering neoadjuvant therapy to all patients with STS together with future perspectives.
Latest evidence and clinical implications
Patient risk stratification
Identification of patients who are at high risk for relapse and may respond to currently available treatments can maximize effectiveness of preoperative chemotherapy and spare treatment-related adverse events in patients who are unlikely to respond. Staging of STS, which is the most important means to stratify patient prognosis, has been a longstanding issue. The sixth and seventh edition of the AJCC staging manual account for only a limited amount of prognostic information, including tumour size, grade, and location with respect to the superficial muscular fascia. The clinical value of this classification has been questioned, particularly for retroperitoneal tumours,18 as these tumours are always deep seated and the 5cm size cutoff does not apply as these tumours are unlikely to be diagnosed when small. Experts agree that prognostic tools need to account for differences across sarcoma histologies and primary tumour sites.19,20
The recently released eighth edition of the AJCC TNM staging manual for STS represents an unprecedented change in risk stratification of patients with sarcomas.21 The manual includes at least two major changes: specific staging for head and neck, limb and trunk, and retroperitoneal sarcomas, and inclusion of a nomogram for the prognostic assessment of patients with retroperitoneal sarcomas.22 Defining T-stage categories according to size is now tailored for the different primary tumour sites. Head and neck sarcomas are classified as T1, T2, T3, and T4 when their size is 2 cm or less, 2–4 cm, and greater than 4cm, and involving adjacent structures. Retroperitoneal, extremity, and trunk tumour sarcomas are classified as T1, T2, T3, and T4 when their size is 5 cm or less, 5–10 cm, 10–15 cm, and greater than 15 cm. However, these cutoff values have some limitations. Despite acknowledging that size is a prognostic factor, they are arbitrary and most of patients with retroperitoneal STS have tumours larger than 15 cm.
These issues are overcome by predictive and prognostic tools, which have advantages over the standard AJCC TNM staging system as they inform physicians about the choice of treatment to be performed on a single patient, based on risk of disease progression and, ultimately, death.23 Nomograms are becoming widely used among surgical and medical oncologists dealing with sarcomas.24 The first nomogram, which was developed in 2002 by Kattan et al.25 and subsequently validated,26–28 predicts the likelihood of being alive within 12 years from initial surgery. However, this tool included histological entities, such as malignant fibrous histiocytoma, that are no longer included in World Health Organization sarcoma classification and all tumour sites. Since then, several nomograms have been developed, including histology-specific nomograms for liposarcomas29 and synovial sarcomas,30 site-specific nomograms for both extremity31,32 and retroperitoneal sarcomas,22,33,34 and also uterine leiomyosarcomas.35 Among these nomograms, the ‘Sarculator’ (http://www.sarculator.com/) is a freely available online resource with embedded nomograms for retroperitoneal22,36 and extremity32 sarcomas. This prognostic tool predicts distant metastasis-free and overall survival at 5 and 10 years after surgery for the primary tumour for extremity STS and at 7 years for retroperitoneal tumours. Remarkably, information not currently considered in the AJCC TNM staging manual are included in the Sarculator. Additional factors included in this prognostic tool for retroperitoneal tumours are age, completeness of resection, histology, and multifocality. Interestingly, there is a U-shaped association between tumour size and prognosis, with very large tumours behaving as smaller sarcomas. This reflects the analysed population, which included patients who underwent surgery and were distant metastasis free. Clearly, when retroperitoneal sarcomas are amenable to surgical resection despite being large, tumour biology is likely to be indolent as a more aggressive lesion will already have metastasized.37 In the nomogram for extremity sarcomas, STS histology is an independent prognostic factor, while completeness of surgical resection does not correlate with survival. Also, age is a predictor only for overall survival and is not included in the prediction for distant metastasis-free survival. Remarkably, there is a greater influence of tumour histology compared with the retroperitoneal sarcoma nomogram. Vascular sarcomas, leiomyosarcomas, and synovial sarcomas show the highest risk of progression to distant sites and patient death. Conversely, myxoid liposarcomas, dedifferentiated liposarcomas, undifferentiated pleomorphic sarcomas, and myxofibrosarcomas are associated with better outcomes. Also, completeness of surgery is not relevant and a linear relationship between size and survival is observed.
There are challenges for using these models when selecting patients for neoadjuvant therapies. First, they are based on features available after the pathological examination of the whole tumour. A nomogram for synovial sarcomas, which are among the most chemosensitive sarcoma histologies, is accurate in identifying patients who may benefit from cytotoxic chemotherapy based on preoperative biopsy.30 Also, the association between higher risk of metastasis and greater response to chemoradiation is still to be proven. It seems that nomograms can predict the pathological response after chemotherapy in patients with retroperitoneal sarcomas, although effectiveness of this treatment modality is still unproven for these patients.38 Although there are limitations in applying these models to neoadjuvant therapy, it can give prognostic information and identify patients who could benefit from adjuvant therapy, and by extension, neoadjuvant therapy as well.
Neoadjuvant chemotherapy
The effectiveness of systemic perioperative chemotherapy for patients with high-risk STS has been widely debated.12,39 Major phase II–III trials are reported in Table 1. An American trial randomized patients with large, high-grade, extremity STS to a regimen of preoperative chemotherapy consisting of mesna, adriamycin (doxorubicin), ifosfamide, and dacarbazine (MAID), and followed by resection and postoperative chemotherapy with or without radiotherapy (44 Gy).40 This trial included 340 patients with either metastatic or unresectable soft tissue and bone sarcomas and showed that an improved response rate may be relevant in high-grade, borderline resectable lesions or pulmonary metastases, particularly in younger patients.
Table 1.
Phase II–III randomized controlled trials (RCTs) of neoadjuvant chemotherapy for soft tissue sarcomas.
| Study | Inclusion criteria | Treatment arms | No. of pts |
CR/PR (%) |
SD (%) |
LRFS (%) |
DRFS (%) |
RFS (%) |
OS (%) |
|---|---|---|---|---|---|---|---|---|---|
| Antman40 | Measurable metastatic or unresectable sarcomas | Arm A: doxorubicin 60 mg/m2 and dacarbazine 1000 mg/m2 days 1–4 every 3 weeks × 3 cycles ± surgery | 170 | 29 (17%) | 69 (41%) | – | – | 2 years: <5% | 3 years: 10% |
| Arm B: doxorubicin 60 mg/m2 and dacarbazine 1000 mg/m2 on days 1–4, ifosfamide 7500 mg/m2 on days 1–3 and mesna 10,000 mg/m2 on days 1–4, every 3 weeks × 3 cycles ± surgery | 170 | 55 (32%) | 49 (29%) | – | – | 2 years: <5% | 3 years: 22% | ||
| Gortzak et al.41 | Size ⩾8 cm or grade 2–3 |
Arm A: doxorubicin 50 mg/m2 day 1, ifosfamide 5 gm/m2 day 1 every 3 weeks × 3 cycles followed by surgery | 67 | 14 (28%) | 26 (53%) | – | – | 5 years: 56% | 5 years: 65% |
| Arm B: surgery alone | 67 | – | – | – | – | 5 years: 52% | 5 years: 64% | ||
| Gronchi et al.42 (updated 201643) | High grade Deep location ⩾5 cm Extremity or trunk tumours |
Arm A: epirubicin 120 mg/m2, ifosfamide 9 gm/m2 × 3 cycles ± XRT followed by surgery | 160 | 36 (23%) | 77 (48%) | 10 years: 91% | 10 years: 66% | 10 years: 56% | 10 years: 64% |
| Arm B: epirubicin 120 mg/m2, ifosfamide 9 gm/m2 × 5 cycles ± XRT, surgery performed after 3 cycles | 161 | 30 (19%) | 92 (57%) | 10 years: 94% | 10 years: 63% | 10 years: 58% | 10 years: 59% | ||
| Gronchi et al.45 | High grade Deep location ⩾5 cm Extremity or trunk tumours (UPS, MPNST, SS, myxoid liposarcomas, and leiomyosarcomas) |
Arm A: epirubicin 120 mg/m2, ifosfamide 9 gm/m2 × 3 cycles ± XRT followed by surgery | 144 | NA | NA | 46 months: 86% | 46 months: 74% | 46 months: 62% | 46 months: 89% |
| Arm B: histology-driven chemotherapy* | 142 | NA | NA | 46 months: 85% | 46 months: 45% | 46 months: 38% | 46 months: 64% |
High-grade myxoid liposarcomas: trabectedin 1.3 mg/m2, given in 24 h continuous infusion day 1 every 3 weeks × 3 cycles; leiomyosarcoma: gemcitabine 1800 mg/m2 on day 1 and dacarbazine 500 mg/m2 on day 1 every 2 weeks; synovial sarcoma (SS): high-dose ifosfamide 14 g/m2 on days 1–14 by means of an external infusion pump every 4 weeks; MPNST: etoposide 150 mg/m2/day, days 1–3 and ifosfamide 3 g/m2/day, days 1–3 every 3 weeks; undifferentiated pleomorphic sarcoma (UPS): gemcitabine 900 mg/m2 on days 1–8 and docetaxel 75 mg/m2 on day 8.
CR, complete response; DRFS, distant relapse-free survival; LRFS, local relapse-free survival; NA, not applicable; OS, overall survival; PR, partial response; RFS, relapse-free survival; SD, stable disease; XRT, radiotherapy.
A European phase III RCT enrolled 134 patients with resectable high-risk primary and recurrent STS. Patients were randomized to either surgery alone or three cycles of doxorubicin (50 mg/m2 intravenous bolus) and ifosfamide (5 g/m2 24 h infusion) before surgery.41 Although this treatment regimen was feasible and did not compromise performance of subsequent surgery, chemotherapy followed by surgical excision was not proven to be more effective than surgery alone (5-year disease-free survival: 56% and 52%, respectively). This trial was burdened by important limitations. First, both primary and recurrent tumours were considered. Also, definition of high risk has been argued as not only were high-grade tumours considered but The French Federation of Comprehensive Cancer Centers (FNCLCC) grade 1 tumours were also included when presenting as a large mass (⩾8 cm). Also, dosages for both doxorubicin and ifosfamide were lower than those used in other studies. Importantly, this RCT closed early due to slow accruals and underlying issues in referral bias to specialized sarcoma centres.
Another study was performed by the Italian and the Spanish Sarcoma Groups (ISG and GEIS).42,43 The design of this study was based on the results of a previous trial run by the former group which investigated adjuvant epirubicin and ifosfamide, which were given using a five-cycle schedule.44 Although the trial was closed in advance because of an early major disease-free survival benefit for patients treated with adjuvant chemotherapy, analysis of data with longer follow up did show a small nonsignificant disease-free and overall survival benefit. Again, patients with several different sarcoma histologies were considered together and pathology review was not consistently performed. Also, drug dose in the last two cycles was significantly reduced. In light of these considerations, a new study was designed comparing three cycles of epirubicin (120 mg/m2) plus ifosfamide (9 g/m2) given preoperatively with five cycles of the same drugs given perioperatively (three neoadjuvant cycles followed by surgery and two further adjuvant cycles).42,43 This study did not identify any survival difference between these two treatment modalities and three chemotherapy cycles given preoperatively were deemed as effective as five cycles. These results were criticized for the lack of a control arm in which patients would have been treated with surgery alone. However, patients in the two treatment arms have a similar prognosis to those treated with adjuvant chemotherapy in the first above-mentioned study by Frustaci et al.,44 suggesting, though indirectly, a potential superiority of combined chemotherapy and surgery over surgery alone. Possible effectiveness of a perioperative treatment is also supported by the observed association between complete response and prognosis, although this evidence could be burdened by a selection bias that may lead to greater tumour response and longer survival independently.
Despite these considerations, this trial did not resolve the long-lasting issue of whether a perioperative treatment can improve survival of patients with high-risk sarcoma. The Italian and Spanish Sarcoma groups went on to design a further randomized trial (ISG-STS-1001) [ClinicalTrials.gov identifier: NCT01710176] which compared epirubicin (60 mg/m2/day on days 1 and 2) and ifosfamide (3 mg/m2/day on days 1, 2, and 3) using a three-cycle schedule which was tested in the previous study with histology-tailored therapeutic regimens for five different STS histologies. Three cycles of the following regimens were administered: gemcitabine (900 mg/m2 on days 1 and 8) plus docetaxel (75 mg/m2 on day 8) in undifferentiated pleomorphic sarcoma; trabectedin (1.3 mg/m2) in high-grade myxoid liposarcoma; high-dose prolonged-infusion ifosfamide (14 g/m2, given in 14 days) in synovial sarcoma; etoposide (150 mg/m2/day on days 1, 2, and 3) plus ifosfamide (3 g/m2/day on days 1, 2, and 3) in malignant peripheral nerve sheath tumours; and gemcitabine (1800 mg/m2 on day 1) plus dacarbazine (500 mg/m2 on day 1) in leiomyosarcoma.
This multicentre study was conducted with the support of the French and Polish Sarcoma Groups and enrolled 286 patients with high-risk STS of the trunk or extremities, from the five above-mentioned histological subtypes, which represent approximately four-fifths of all STS arising in the extremities or trunk wall.45 The study planned to enrol 350 patients, however it was stopped early following a recommendation by the external independent data monitoring committee when the third futility analysis identified a clear disease-free and overall survival benefit for patients treated with three cycles of epirubicin and ifosfamide. The median follow up was 12.3 months and patients treated with standard chemotherapy had statistically significant disease-free (62% versus 38%) and overall (89% versus 64%) survival benefits compared with those who received tailored chemotherapy. Subgroup analysis revealed that mixoyd liposarcoma was the only tumour histology for which histology-driven chemotherapy with trabectedin was as effective as standard chemotherapy. Importantly, disease-free and overall survival of patients in the histology-tailored arm were similar to those of the control arm in the first Italian Sarcoma Group trial comparing adjuvant chemotherapy and observation,44 leading to the conclusion that tailored treatment was likely not effective. Likewise, disease-free survival and overall survival of patients in the standard chemotherapy arm were similar to those of patients in the trial comparing three versus five cycles of cytotoxic chemotherapy.
Neoadjuvant chemoradiation
While neoadjuvant chemotherapy is still not widely accepted among clinicians, the role of radiotherapy in patients with high-risk STS of the extremities and trunk is supported by findings from RCTs, making this treatment standard in high-risk patients.46–49 Radiotherapy and chemotherapy have been combined together to increase the chances of a local response, decreasing the extent of resection and improving the limb salvage rate for STS of the extremities. Also, chemotherapy can enhance the antitumour effect of radiation.
Radiotherapy can be delivered either preoperatively (cumulative dose: 50 Gy) or postoperatively (cumulative dose: 66 Gy). The optimal timing of radiotherapy is debated as preoperative radiation doubles the risk of a wound complication, while postoperative treatment increases the risk of late adverse effects, such as fibrosis, oedema, and joint stiffness.49,50 In the above mentioned RCT, which randomized patients to three cycles of preoperative chemotherapy with epirubicin (120 mg/m2) plus ifosfamide (9 g/m2) alone or in combination with two further postoperative cycles, radiotherapy could be delivered either preoperatively or postoperatively.42,43 Patients treated with these schedules had a cumulative incidence of local recurrence of 17% and 3% in the case of positive and negative margins, respectively, at 5 years.51 Remarkably, in patients who underwent preoperative chemoradiotherapy and had a postoperative positive surgical margin, no local recurrence was observed. These observations are in keeping with nonrandomized studies showing a similar risk of developing local recurrence in patients expected to have a positive margin after neoadjuvant radiotherapy plus surgery, and in those who had negative margins after surgery.52–56 Importantly, these studies also showed that tumours with a positive margin are likely more biologically aggressive than those with a negative margin; these patients are at greater risk of both local and distant relapse, irrespective of surgery extent.54,55 In these patients preoperative radiotherapy can reduce viable tumour cells at the resection margins. Also, these patients are at high risk of metastatic spread and should be considered for preoperative chemotherapy. When a positive margin is reported after radiotherapy plus surgery, a further radiotherapy boost does not seem to lower local recurrence in patients with microscopically positive margins.51,53,57 This is another indirect observation supporting preoperative over postoperative radiotherapy, especially in patients with large tumours in whom resection margins are likely not to be negative.
Chemotherapy can be given alternating with radiation therapy or concurrently. Concomitant administration aims to increase the chances of tumour response and to carry out conservative surgery without jeopardizing local control of the tumour. However, the simultaneous use of radio- and chemotherapy doubles the risk of high-grade thrombocytopenia, which is observed in about one third of patients.58 One in six patients also develop postoperative wound complications. The unclear effectiveness of simultaneous chemo- and radiotherapy balanced against toxicity has led to variations in practice and use of different treatment schedules. For instance, patients with large (8 cm or more) and intermediate- to high-grade sarcomas presenting at some US referral centres undergo two courses of preoperative radiotherapy (22 Gy in 11 fractions delivered in each course with a total of 44 Gy) and three cycles of neoadjuvant chemotherapy with MAID in between.59 Patients treated with this schedule have local control, distant recurrence-free and overall survival of 91%, 64%, and 86%, respectively, after 5 years.60 This treatment modality was also tested in a multicentre prospective phase II study [Radiation Therapy Oncology Group Trial (RTOG) 9514], which enrolled 66 patients.61,62 Grade 3 or higher morbidity was observed in the vast majority of patients (97%), including three treatment-related deaths. Long-term results showed 5-year distant disease-free and overall survival rates of 64% and 71%, respectively. Other combinations have been tested to reduce toxicity compared with MAID, such as intra-arterial adriamycin, intravenous ifosfamide, and a combination of intravenous cisplatin plus adriamycin and ifosfamide, which were administered together with a reduced-dose radiotherapy (28Gy).63 Ifosfamide was found to be the most effective drug to administer together with radiotherapy and patients developing tumour necrosis had less incidence of local recurrence and better survival. Another approach based on preoperative radiotherapy (50 Gy) combined with concurrent escalating doses of gemcitabine plus ifosfamide, which was added for patients treated with definitive radiotherapy or when positive postoperative margins are anticipated, was studied in a phase I trial.64 This schedule achieved 5-year local control, distant metastasis-free, and overall survival rates of 85%, 80%, and 86%, respectively.
Evidence for treatment of retroperitoneal sarcomas with concomitant chemoradiation is lacking. The Italian Sarcoma Group conducted a phase I–II study enrolling 86 patients who received three cycles of high-dose long-infusion ifosfamide (14 g/m2) and radiotherapy which was started on the second chemotherapy cycle and administered up to a total dose of 50.4 Gy.65 Local and distant recurrence occurred in 37% and 26% of patients, respectively, after 5 years, leading to a disease-free and overall survival of 44% and 59%, respectively. Although the results were encouraging, only two-thirds of enrolled participants completed the preoperative treatment, likely reflecting the burden of such a treatment modality in a population who often presented with significant comorbidities and low performance status. A retrospective study compared the outcomes of patients treated with neoadjuvant chemotherapy and surgery alone for retroperitoneal sarcomas.66 Length of hospital stay, rate of readmission, and rate of reoperation for complications were similar for patients treated with these two approaches. However, three postoperative deaths occurred in patients treated with neoadjuvant chemotherapy. Overall, considering the risk associated with chemotherapy in these patients and the observed incidence of local recurrences, research is focusing on radiotherapy. A population-based study showed an association between performance of radiotherapy and better survival in patients with retroperitoneal sarcomas.67 An RCT comparing preoperative radiotherapy followed by surgery and surgery alone (EORTC-STRASS) [ClinicalTrials.gov identifier: NCT01344018] will offer more definitive data on the role of radiotherapy in treating these tumours.
Newly introduced effective drugs for the treatment of metastatic tumours are going to be tested in the neoadjuvant setting concurrently with radiotherapy. For instance, pazopanib, an orally available tyrosine kinase inhibitor, is being tested in combination with radiotherapy in a phase II nonrandomized study [ClinicalTrials.gov identifier: NCT02575066] and in a phase II–III study randomizing patients preoperatively to radiation plus pazopanib or to radiation alone [ClinicalTrials.gov identifier: NCT02180867]. Also, radiotherapy can enhance the effectiveness of immunotherapy, particularly checkpoint inhibitors.68 Some patients with metastatic tumours who undergo radiotherapy develop tumour responses not only at the site of treatment but also on other tumour deposits, generating the so-called ‘abscopal effect’.69 The immune mechanisms underlying these effects has been better described, suggesting that combinations of radiotherapy and immune therapy could impact patient outcomes.70
Hyperthermia and neoadjuvant chemotherapy
Regional hyperthermia is another therapeutic strategy for improving locoregional control in patients with several malignancies, such as recurrent breast cancer,71 melanoma,72 cervical cancer,73 and malignant germ-cell tumours.74 In STS, the effectiveness of neoadjuvant chemotherapy can be enhanced when patients are also treated with hyperthermia.75,76 Hyperthermia sensitizes tumour cells by ionizing radiation, which acts as a pleiotropic damaging agent altering protein structures and influencing the DNA damage response.77 A phase III RCT, which compared neoadjuvant chemotherapy with etoposide, doxorubicin, and ifosfamide alone or in combination with hyperthermia, showed patients with high-risk primary sarcomas treated with both modalities are at lower risk of disease progression (76% versus 61% after 2 years), which was the primary endpoint of this study, compared with those who underwent only chemotherapy.76 Also, the hyperthermia doubled tumour response (29% versus 13%). However, overall survival did not differ between the two groups. A subgroup analysis of patients with retroperitoneal and abdominal sarcomas confirmed the effectiveness of improving local tumour control (56% versus 45% after 5 years) and disease-free survival (34% versus 27% after 5 years) in patients who had macroscopically complete tumour resection.78 These results were recently updated by analysing 9-year follow-up data and presented in abstract form.79 A significantly prolonged overall survival was observed in patients receiving regional hyperthermia compared with patients receiving only chemotherapy (63% versus 51% after 5 years). Despite these positive results, the advantages of a combination of neoadjuvant chemotherapy and hyperthermia have not been confirmed yet by other trials.
Open issues
Preoperative histology characterization at core biopsy
Pathological examination of core biopsy can lead to accurate diagnosis for extremity and trunk sarcomas.80 However, core biopsy does not seem to be accurate for defining tumour differentiation and grade of lipomatous tumours seated in the retroperitoneum, which are characterized by large size and significant heterogeneity.81
Assessment of tumour response
The evaluation of tumour response after neoadjuvant treatments is another unresolved issue. Radiological imaging is needed after neoadjuvant therapies in order to formulate an adequate surgical plan. Also, imaging can offer significant information on the effect of neoadjuvant treatments. Response Evaluation Criteria In Solid Tumors (RECIST) criteria, which are based on unidimensional tumour measurement, selection of target lesions, and a threshold for assignment of objective progression, are the most widely used means to evaluate tumour response.82 However, they are not always accurate in evaluating tumour response when molecular target agents are used, such as in the case of gastrointestinal stromal tumours.83 In these soft-tissue tumours the effect of targeted therapies can result in different modifications compared with standard cytotoxic chemotherapy. Standard chemotherapy results in tumour shrinkage, while targeted drugs also generate changes in tumour density. Importantly, these differences are seen in some sarcoma histologies treated with cytotoxic chemotherapy (Figures 1 and 2).84,85 For instance, in synovial sarcoma treated with epirubicin and ifosfamide, tumour attenuation on a contrast-enhanced computed tomography (CT) scan and tumour contrast enhancement on magnetic resonance imaging adds predictive information to changes in tumour size.84 Another useful tool to predict response to treatment for STS is positron emission tomography (PET) CT. Standardized Uptake Value (SUV) values before and after neoadjuvant chemotherapy have been associated with the chance of developing a tumour response.86,87 However, 2-deoxy-2[F-18]fluoro-D-glucose (FDG) uptake varies across sarcoma histologies and more research is needed to identify when PET CT can significantly add to the management of these patients.88
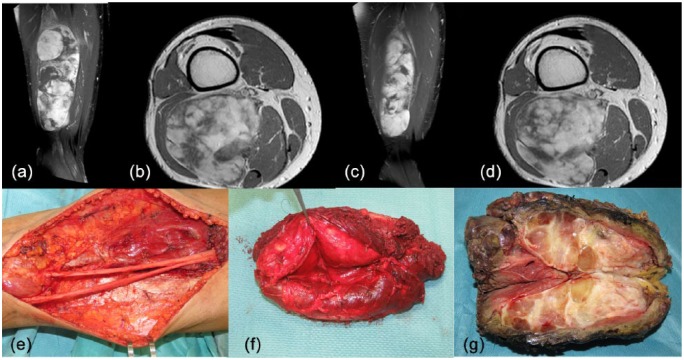
Figure 1.
A 38-year-old man was diagnosed with a 8 × 6 × 21 cm mass in his right posterior thigh (a, b) [contrast-enhanced magnetic resonance imaging (MRI), TW1 weighted sequences]. Percutaneous core needle biopsy revealed a high-grade round-cell myxoid liposarcoma (round cells component > 60%). This patient was treated with three cycles of epirubicin (120 mg/m2) and ifosfamide (9000 mg/m2) and concomitant radiotherapy (50 Gy in 25 fractions). After neoadjuvant chemoradiation, contrast-enhanced MRI showed dimensional changes (8 × 2 × 16 cm) and modification in pattern of contrast enhancement, suggesting a tissue response (c, d). Surgery involved a wide excision of the posterior thigh with the sciatic nerve dissected off the tumour (e–g). The pathology report showed two small areas with hypercellularity (0.5 and 1.5 cm, respectively) and negative surgical margins.
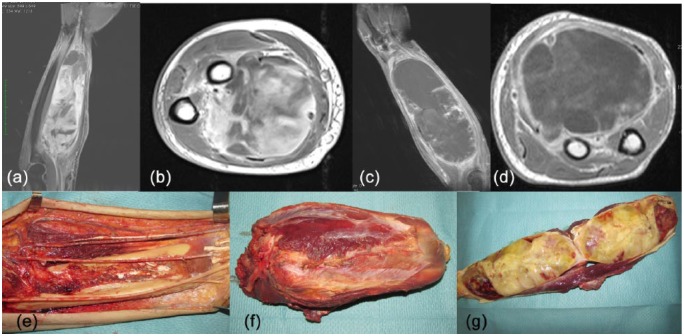
Figure 2.
A 58-year-old man was diagnosed with a 6 × 20 cm mass in his left volar forearm. Percutaneous core needle biopsy revealed a high-grade myxofibrosarcoma (a, b) [contrast-enhanced magnetic resonance imaging (MRI), TW1 weighted sequences]. This patient was treated with three cycles of epirubicin (120 mg/m2) and ifosfamide (9000 mg/m2) and concomitant radiotherapy (50 Gy in 25 fractions). MRI showed an increase in tumour dimension and a strong reduction of tissue contrast enhancement, suggesting a tissue response (c, d). Surgery involved a wide excision of the posterior forearm (e). The tumour was resected together with the median nerve which was completely surrounded by the tumour (f, g). The pathology report showed significant presence of necrosis (70% of the tumour mass) and limited residual tumour (30%).
Pathological examination provides definitive assessment of tumour response. However, guidance on how this evaluation should be performed is lacking and classification for characterizing tumour necrosis and its patterns has not been established for STS. Existing data on the association between necrosis and survival are conflicting,63,89,90 and further research is needed to improve prediction of prognosis of patients after neoadjuvant chemotherapy with or without radiation plus surgery. Secondary analyses of the ISG-STS-1001 are expected to shed light on the assessment of tumour response using imaging and pathology evaluation.
Histology-driven chemotherapy and patient selection
The effect of different chemotherapeutics across sarcoma histologies also needs further research. Several histotypes, such as alveolar soft part sarcoma,91 clear cell sarcoma,92 and classical-type epithelioid sarcoma,93 are among the most chemoresistant sarcomas. However, other sarcomas are considered more likely to respond to chemotherapy, such as synovial sarcoma and high-grade myxoid liposarcoma (Figure 1), making them candidates for neoadjuvant treatments. Synovial sarcomas are among the most chemosensitive sarcomas,94,95 especially ifosfamide-containing regimens in the metastatic setting.96 In light of these findings, the ISG-STS-1001 trial randomized patients with synovial sarcoma to epirubicin plus ifosfamide or high-dose ifosfamide. Patients treated with high-dose ifosfamide did worse [hazard ratio (HR) 1.85, 95% confidence interval (CI) 0.56–5.22], although this difference was not significant. Despite the observation of a better chemosensitivity for synovial sarcomas, studies showed that the effect of cytotoxic chemotherapy may be relatively small. The above-mentioned nomogram for patients with synovial sarcoma undergoing resection with curative intent showed that treatment with doxorubicin plus ifosfamide was associated with a statistically superior 3-year survival, although these improvements were lost over time.30 An European Organization for Research and Treatment of Cancer (EORTC) study pooled together the data of 313 patients with synovial sarcomas treated in 15 different prospective trials and showed that these patients had a significantly higher chance of benefitting from chemotherapy compared with those having other sarcomas (28% and 19% response rate, respectively). This translated into a small, although statistically significant, improvement in survival (progression-free survival: 6 versus 4 months, respectively; overall survival: 15 versus 12 months, respectively).97
Myxoid liposarcoma, which is defined by a DDIT3-FUS or DDIT3-EWSR1 gene fusion, is characterized by good outcomes, although high-grade tumours (i.e. round cell component >5%) showed more aggressive behaviour.98 In a retrospective study, virtually all patients with myxoid liposarcoma treated with chemotherapy survived 5 years after surgery.99 Trabectedin, which blocks DNA binding of the oncogenic transcription factor FUS-CHOP,100,101 is an effective agent in this sarcoma subtype both in the metastatic102 and neoadjuvant103 setting. The above-mentioned ISG-STS-1001 trial compared standard epirubicin plus ifosfamide and trabectidine for these patients. Interestingly, the two regimens showed similar effectiveness (HR 1.03; 95% CI 0.24–4.39), which favours trabectedin for its more acceptable toxicity profile. These findings need to be confirmed in a larger prospective series and this study is going to be reopened enrolling only patients with high-grade myxoid liposarcomas.
Undifferentiated pleomorphic sarcoma has been considered a chemoresistant histology with unfavourable prognosis, particularly when these tumours are located in the retroperitoneum.104 In a recent population-based analysis, prognosis of patients with undifferentiated pleomorphic sarcoma was significantly better when adjuvant/neoadjuvant chemotherapy was used (median survival 78 and 49 months, respectively).14 Also, these tumours harbour a significant genomic instability,105,106 suggesting they may be candidates for newly introduced immune checkpoint inhibitors.107 Overall, certain histologies lend themselves to tailored therapy such as Myxoid liposarcoma (MLS), however the ISG-STS-1001 study confirmed that an anthracycline with ifosfamide for all other subtypes is preferred.
Conclusion
Significant improvements in patient risk stratification through a new AJCC TNM classification and nomograms can better stratify the risk of patients with primary STS. This is of great importance since neoadjuvant epirubicin and ifosfamide showed effectiveness in locally advanced high-risk primary sarcomas of the trunk and extremities. Better prognostic tools, a wider array of chemotherapy options, and better predictive biomarkers are needed for patients with high-risk sarcomas.
Acknowledgments
SP is attending the PhD program in Clinical and Experimental Oncology and Immunology of the University of Padova, Padova, Italy.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Sandro Pasquali, Sarcoma Service, Department of Surgery, Fondazione IRCCS Istituto Nazionale dei Tumori, Milano, Italy.
Alessandro Gronchi, Sarcoma Service, Department of Surgery, Fondazione IRCCS Istituto Nazionale dei Tumori, Via G Venezian 1, 20013 Milano, Italy.
References
- 1. Fletcher CDM, Bridge JA, Hogendoorn P, et al. WHO classification of tumours of soft tissue and bone. Pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press, 2013. [Google Scholar]
- 2. Jo VY, Doyle LA. Refinements in sarcoma classification in the current 2013 World Health Organization Classification of Tumours of Soft Tissue and Bone. Surg Oncol Clin N Am 2016; 25: 621–643. [DOI] [PubMed] [Google Scholar]
- 3. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016; 66: 271–289. [DOI] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 5. Brennan MF, Antonescu CR, Moraco N, et al. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg 2014; 260: 416–421; discussion 21–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014; 25(Suppl 3): iii102–iii112. [DOI] [PubMed] [Google Scholar]
- 7. Cable MG, Randall RL. Extremity soft tissue sarcoma: tailoring resection to histologic subtype. Surg Oncol Clin N Am 2016; 25: 677–695. [DOI] [PubMed] [Google Scholar]
- 8. Gladdy RA, Gupta A, Catton CN. Retroperitoneal sarcoma: fact, opinion, and controversy. Surg Oncol Clin N Am 2016; 25: 697–711. [DOI] [PubMed] [Google Scholar]
- 9. Larrier NA, Czito BG, Kirsch DG. Radiation therapy for soft tissue sarcoma: indications and controversies for neoadjuvant therapy, adjuvant therapy, intraoperative radiation therapy, and brachytherapy. Surg Oncol Clin N Am 2016; 25: 841–860. [DOI] [PubMed] [Google Scholar]
- 10. Jacobs AJ, Michels R, Stein J, et al. Improvement in overall survival from extremity soft tissue sarcoma over twenty years. Sarcoma 2015; 2015: 279601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gronchi A, Miceli R, Colombo C, et al. Primary extremity soft tissue sarcomas: outcome improvement over time at a single institution. Ann Oncol 2011; 22: 1675–1681. [DOI] [PubMed] [Google Scholar]
- 12. Casali PG. Adjuvant chemotherapy for soft tissue sarcoma. Am Soc Clin Oncol Educ Book. 2015: e629–e633. [DOI] [PubMed] [Google Scholar]
- 13. Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008; 113: 573–581. [DOI] [PubMed] [Google Scholar]
- 14. Movva S, von Mehren M, Ross EA, et al. Patterns of chemotherapy administration in high-risk soft tissue sarcoma and impact on overall survival. J Natl Compr Canc Netw 2015; 13: 1366–1374. [DOI] [PubMed] [Google Scholar]
- 15. Colombo C, Randall RL, Andtbacka RH, et al. Surgery in soft tissue sarcoma: more conservative in extremities, more extended in the retroperitoneum. Expert Rev Anticancer Ther 2012; 12: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 16. Saponara M, Stacchiotti S, Casali PG, et al. (Neo)adjuvant treatment in localised soft tissue sarcoma: the unsolved affair. Eur J Cancer 2016; 70: 1–11. [DOI] [PubMed] [Google Scholar]
- 17. Radaelli S, Stacchiotti S, Casali PG, et al. Emerging therapies for adult soft tissue sarcoma. Expert Rev Anticancer Ther 2014; 14: 689–704. [DOI] [PubMed] [Google Scholar]
- 18. van Dalen T, Hennipman A, Van Coevorden F, et al. Evaluation of a clinically applicable post-surgical classification system for primary retroperitoneal soft-tissue sarcoma. Ann Surg Oncol 2004; 11: 483–490. [DOI] [PubMed] [Google Scholar]
- 19. Maki RG, Moraco N, Antonescu CR, et al. Toward better soft tissue sarcoma staging: building on American Joint Committee on Cancer Staging systems versions 6 and 7. Ann Surg Oncol 2013; 20: 3377–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anaya DA, Lahat G, Wang X, et al. Establishing prognosis in retroperitoneal sarcoma: a new histology-based paradigm. Annals of Surgical Oncology 2009; 16: 667–675. [DOI] [PubMed] [Google Scholar]
- 21. Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. New York: Springer-Verlag, 2017. [Google Scholar]
- 22. Gronchi A, Miceli R, Shurell E, et al. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J Clin Oncol 2013; 31: 1649–1655. [DOI] [PubMed] [Google Scholar]
- 23. Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008; 26: 1364–1370. [DOI] [PubMed] [Google Scholar]
- 24. Massarweh NN, Dickson PV, Anaya DA. Soft tissue sarcomas: staging principles and prognostic nomograms. J Surg Oncol 2015; 111: 532–539. [DOI] [PubMed] [Google Scholar]
- 25. Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol 2002; 20: 791–796. [DOI] [PubMed] [Google Scholar]
- 26. Eilber FC, Brennan MF, Eilber FR, et al. Validation of the postoperative nomogram for 12-year sarcoma-specific mortality. Cancer 2004; 101: 2270–2275. [DOI] [PubMed] [Google Scholar]
- 27. Mariani L, Miceli R, Kattan MW, et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer 2005; 103: 402–408. [DOI] [PubMed] [Google Scholar]
- 28. Bagaria SP, Wagie AE, Gray RJ, et al. Validation of a soft tissue sarcoma nomogram using a national cancer registry. Ann Surg Oncol 2015; 22(Suppl 3): S398–S403. [DOI] [PubMed] [Google Scholar]
- 29. Dalal KM, Kattan MW, Antonescu CR, et al. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg 2006; 244: 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Canter RJ, Qin LX, Maki RG, et al. A synovial sarcoma-specific preoperative nomogram supports a survival benefit to ifosfamide-based chemotherapy and improves risk stratification for patients. Clin Cancer Res 2008; 14: 8191–8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cahlon O, Brennan MF, Jia X, et al. A postoperative nomogram for local recurrence risk in extremity soft tissue sarcomas after limb-sparing surgery without adjuvant radiation. Ann Surg 2012; 255: 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Callegaro D, Miceli R, Bonvalot S, et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis. Lancet Oncol 2016; 17: 671–680. [DOI] [PubMed] [Google Scholar]
- 33. Ardoino I, Miceli R, Berselli M, et al. Histology-specific nomogram for primary retroperitoneal soft tissue sarcoma. Cancer 2010; 116: 2429–2436. [DOI] [PubMed] [Google Scholar]
- 34. Anaya DA, Lahat G, Wang X, et al. Postoperative nomogram for survival of patients with retroperitoneal sarcoma treated with curative intent. Ann Oncol 2010; 21: 397–402. [DOI] [PubMed] [Google Scholar]
- 35. Zivanovic O, Jacks LM, Iasonos A, et al. A nomogram to predict postresection 5-year overall survival for patients with uterine leiomyosarcoma. Cancer 2012; 118: 660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raut CP, Miceli R, Strauss DC, et al. External validation of a multi-institutional retroperitoneal sarcoma nomogram. Cancer 2016; 122: 1417–1424. [DOI] [PubMed] [Google Scholar]
- 37. Pasquali S, Gronchi A, Strauss D, et al. Resectable extra-pleural and extra-meningeal solitary fibrous tumours: a multi-centre prognostic study. Eur J Surg Oncol 2016; 42: 1064–1070. [DOI] [PubMed] [Google Scholar]
- 38. Donahue TR, Kattan MW, Nelson SD, et al. Evaluation of neoadjuvant therapy and histopathologic response in primary, high-grade retroperitoneal sarcomas using the sarcoma nomogram. Cancer 2010; 116: 3883–3891. [DOI] [PubMed] [Google Scholar]
- 39. D’Adamo D. Is adjuvant chemotherapy useful for soft-tissue sarcomas? Lancet Oncol 2012; 13: 968–970. [DOI] [PubMed] [Google Scholar]
- 40. Antman K, Crowley J, Balcerzak SP, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol 1993; 11: 1276–1285. [DOI] [PubMed] [Google Scholar]
- 41. Gortzak E, Azzarelli A, Buesa J, et al. A randomised phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer 2001; 37: 1096–1103. [DOI] [PubMed] [Google Scholar]
- 42. Gronchi A, Frustaci S, Mercuri M, et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol 2012; 30: 850–856. [DOI] [PubMed] [Google Scholar]
- 43. Gronchi A, Stacchiotti S, Verderio P, et al. Short, full-dose adjuvant chemotherapy (CT) in high-risk adult soft tissue sarcomas (STS): long-term follow-up of a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. Ann Oncol 2016; 27(12): 2283–2288. [DOI] [PubMed] [Google Scholar]
- 44. Frustaci S, Gherlinzoni F, De Paoli A, et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol 2001; 19: 1238–1247. [DOI] [PubMed] [Google Scholar]
- 45. Gronchi A, Ferrari S, Quagliuolo V, et al. Neoadjuvant chemotherapy in high-risk soft tissue sarcomas: a randomised clinical trial from the Italian Sarcoma Group (ISG), the Spanish Sarcoma Group (GEIS), the French Sarcoma Group (FSG) and the Polish Sarcoma Group (PSG). Lancet Oncol 2017. [DOI] [PubMed] [Google Scholar]
- 46. Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982; 196: 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 1998; 16: 197–203. [DOI] [PubMed] [Google Scholar]
- 48. Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 1996; 14: 859–868. [DOI] [PubMed] [Google Scholar]
- 49. O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 2002; 359: 2235–2241. [DOI] [PubMed] [Google Scholar]
- 50. Davis AM, O’Sullivan B, Bell RS, et al. Function and health status outcomes in a randomized trial comparing preoperative and postoperative radiotherapy in extremity soft tissue sarcoma. J Clin Oncol 2002; 20: 4472–4477. [DOI] [PubMed] [Google Scholar]
- 51. Gronchi A, Verderio P, De Paoli A, et al. Quality of surgery and neoadjuvant combined therapy in the ISG-GEIS trial on soft tissue sarcomas of limbs and trunk wall. Ann Oncol 2013; 24: 817–823. [DOI] [PubMed] [Google Scholar]
- 52. Dagan R, Indelicato DJ, McGee L, et al. The significance of a marginal excision after preoperative radiation therapy for soft tissue sarcoma of the extremity. Cancer 2012; 118: 3199–3207. [DOI] [PubMed] [Google Scholar]
- 53. Al Yami A, Griffin AM, Ferguson PC, et al. Positive surgical margins in soft tissue sarcoma treated with preoperative radiation: is a postoperative boost necessary? Int J Radiat Oncol Biol Phys 2010; 77: 1191–1197. [DOI] [PubMed] [Google Scholar]
- 54. Grimer RJ. On the effect of setting of a positive surgical margin in soft tissue sarcoma. Cancer 2014; 120: 2803–2805. [DOI] [PubMed] [Google Scholar]
- 55. O’Donnell PW, Griffin AM, Eward WC, et al. The effect of the setting of a positive surgical margin in soft tissue sarcoma. Cancer 2014; 120: 2866–2875. [DOI] [PubMed] [Google Scholar]
- 56. Gerrand CH, Wunder JS, Kandel RA, et al. Classification of positive margins after resection of soft-tissue sarcoma of the limb predicts the risk of local recurrence. J Bone Joint Surg Br 2001; 83: 1149–1155. [DOI] [PubMed] [Google Scholar]
- 57. Pan E, Goldberg SI, Chen YL, et al. Role of post-operative radiation boost for soft tissue sarcomas with positive margins following pre-operative radiation and surgery. J Surg Oncol 2014; 110: 817–822. [DOI] [PubMed] [Google Scholar]
- 58. Palassini E, Ferrari S, Verderio P, et al. Feasibility of preoperative chemotherapy with or without radiation therapy in localized soft tissue sarcomas of limbs and superficial trunk in the Italian sarcoma group/Grupo Espanol de Investigacion en sarcomas randomized clinical trial: three versus five cycles of full-dose epirubicin plus ifosfamide. J Clin Oncol 2015; 33: 3628–3634. [DOI] [PubMed] [Google Scholar]
- 59. DeLaney TF, Spiro IJ, Suit HD, et al. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys 2003; 56: 1117–1127. [DOI] [PubMed] [Google Scholar]
- 60. Look Hong NJ, Hornicek FJ, Harmon DC, et al. Neoadjuvant chemoradiotherapy for patients with high-risk extremity and truncal sarcomas: a 10-year single institution retrospective study. Eur J Cancer 2013; 49: 875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kraybill WG, Harris J, Spiro IJ, et al. Long-term results of a phase 2 study of neoadjuvant chemotherapy and radiotherapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. Cancer 2010; 116: 4613–4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kraybill WG, Harris J, Spiro IJ, et al. Phase II study of neoadjuvant chemotherapy and radiation therapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. J Clin Oncol 2006; 24: 619–625. [DOI] [PubMed] [Google Scholar]
- 63. Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol 2001; 19: 3203–3209. [DOI] [PubMed] [Google Scholar]
- 64. Tseng WW, Zhou S, To CA, et al. Phase 1 adaptive dose-finding study of neoadjuvant gemcitabine combined with radiation therapy for patients with high-risk extremity and trunk soft tissue sarcoma. Cancer 2015; 121: 3659–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gronchi A, De Paoli A, Dani C, et al. Preoperative chemo-radiation therapy for localised retroperitoneal sarcoma: a phase I–II study from the Italian Sarcoma Group. Eur J Cancer 2014; 50: 784–792. [DOI] [PubMed] [Google Scholar]
- 66. Meric F, Milas M, Hunt KK, et al. Impact of neoadjuvant chemotherapy on postoperative morbidity in soft tissue sarcomas. J Clin Oncol 2000; 18: 3378–3383. [DOI] [PubMed] [Google Scholar]
- 67. Nussbaum DP, Rushing CN, Lane WO, et al. Preoperative or postoperative radiotherapy versus surgery alone for retroperitoneal sarcoma: a case-control, propensity score-matched analysis of a nationwide clinical oncology database. Lancet Oncol 2016; 17: 966–975. [DOI] [PubMed] [Google Scholar]
- 68. Esposito A, Criscitiello C, Curigliano G. Immune checkpoint inhibitors with radiotherapy and locoregional treatment: synergism and potential clinical implications. Curr Opin Oncol 2015; 27: 445–451. [DOI] [PubMed] [Google Scholar]
- 69. Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366: 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin 2016. [DOI] [PubMed] [Google Scholar]
- 71. Jones EL, Oleson JR, Prosnitz LR, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 2005; 23: 3079–3085. [DOI] [PubMed] [Google Scholar]
- 72. Overgaard J, Gonzalez D, Hulshof MC, et al. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet 1995; 345: 540–543. [DOI] [PubMed] [Google Scholar]
- 73. van der Zee J, Gonzalez D, van Rhoon GC, et al. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet. 2000; 355: 1119–1125. [DOI] [PubMed] [Google Scholar]
- 74. Wessalowski R, Schneider DT, Mils O, et al. Regional deep hyperthermia for salvage treatment of children and adolescents with refractory or recurrent non-testicular malignant germ-cell tumours: an open-label, non-randomised, single-institution, phase 2 study. Lancet Oncol 2013; 14: 843–852. [DOI] [PubMed] [Google Scholar]
- 75. Issels RD, Abdel-Rahman S, Wendtner C, et al. Neoadjuvant chemotherapy combined with regional hyperthermia (RHT) for locally advanced primary or recurrent high-risk adult soft-tissue sarcomas (STS) of adults: long-term results of a phase II study. Eur J Cancer 2001; 37: 1599–1608. [DOI] [PubMed] [Google Scholar]
- 76. Issels RD, Lindner LH, Verweij J, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol 2010; 11: 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kaur P, Hurwitz MD, Krishnan S, et al. Combined hyperthermia and radiotherapy for the treatment of cancer. Cancers (Basel) 2011; 3: 3799–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Angele MK, Albertsmeier M, Prix NJ, et al. Effectiveness of regional hyperthermia with chemotherapy for high-risk retroperitoneal and abdominal soft-tissue sarcoma after complete surgical resection: a subgroup analysis of a randomized phase-III multicenter study. Ann Surg 2014; 260: 749–754; discussion 54–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Issels RD, Lindner LH, Ghadjar P, et al. Improved overall survival by adding regional hyperthermia to neoadjuvant chemotherapy in patients with localized high-risk soft tissue sarcoma (HR-STS): long-term outcomes of the EORTC 62961/ESHO randomized phase III study. ESMO Meeting Wien: Ann Oncol, 2015. [Google Scholar]
- 80. Hoeber I, Spillane AJ, Fisher C, Thomas JM. Accuracy of biopsy techniques for limb and limb girdle soft tissue tumors. Ann Surg Oncol 2001; 8: 80–87. [DOI] [PubMed] [Google Scholar]
- 81. Ikoma N, Torres KE, Somaiah N, et al. Accuracy of preoperative percutaneous biopsy for the diagnosis of retroperitoneal liposarcoma subtypes. Ann Surg Oncol 2015; 22: 1068–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–216. [DOI] [PubMed] [Google Scholar]
- 83. Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 2007; 25: 1753–1759. [DOI] [PubMed] [Google Scholar]
- 84. Stacchiotti S, Collini P, Messina A, et al. High-grade soft-tissue sarcomas: tumor response assessment–pilot study to assess the correlation between radiologic and pathologic response by using RECIST and Choi criteria. Radiology 2009; 251: 447–456. [DOI] [PubMed] [Google Scholar]
- 85. Taieb S, Saada-Bouzid E, Tresch E, et al. Comparison of response evaluation criteria in solid tumours and Choi criteria for response evaluation in patients with advanced soft tissue sarcoma treated with trabectedin: a retrospective analysis. Eur J Cancer 2015; 51: 202–209. [DOI] [PubMed] [Google Scholar]
- 86. Fendler WP, Lehmann M, Todica A, et al. PET response criteria in solid tumors predicts progression-free survival and time to local or distant progression after chemotherapy with regional hyperthermia for soft-tissue sarcoma. J Nucl Med 2015; 56: 530–537. [DOI] [PubMed] [Google Scholar]
- 87. Tateishi U, Kawai A, Chuman H, et al. PET/CT allows stratification of responders to neoadjuvant chemotherapy for high-grade sarcoma: a prospective study. Clin Nucl Med 2011; 36: 526–532. [DOI] [PubMed] [Google Scholar]
- 88. Becher S, Oskouei S. PET Imaging in Sarcoma. Orthop Clin North Am 2015; 46: 409–415, xi. [DOI] [PubMed] [Google Scholar]
- 89. Mullen JT, Hornicek FJ, Harmon DC, et al. Prognostic significance of treatment-induced pathologic necrosis in extremity and truncal soft tissue sarcoma after neoadjuvant chemoradiotherapy. Cancer 2014; 120: 3676–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. MacDermed DM, Miller LL, Peabody TD, et al. Primary tumor necrosis predicts distant control in locally advanced soft-tissue sarcomas after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys 2010; 76: 1147–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Reichardt P, Lindner T, Pink D, et al. Chemotherapy in alveolar soft part sarcomas. What do we know? Eur J Cancer 2003; 39: 1511–1516. [DOI] [PubMed] [Google Scholar]
- 92. Jones RL, Constantinidou A, Thway K, et al. Chemotherapy in clear cell sarcoma. Med Oncol 2011; 28: 859–863. [DOI] [PubMed] [Google Scholar]
- 93. Guzzetta AA, Montgomery EA, Lyu H, et al. Epithelioid sarcoma: one institution’s experience with a rare sarcoma. J Surg Res 2012; 177: 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Spillane AJ, A’Hern R, Judson IR, et al. Synovial sarcoma: a clinicopathologic, staging, and prognostic assessment. J Clin Oncol 2000; 18: 3794–3803. [DOI] [PubMed] [Google Scholar]
- 95. Eilber FC, Brennan MF, Eilber FR, et al. Chemotherapy is associated with improved survival in adult patients with primary extremity synovial sarcoma. Ann Surg 2007; 246: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rosen G, Forscher C, Lowenbraun S, et al. Synovial sarcoma. Uniform response of metastases to high dose ifosfamide. Cancer 1994; 73: 2506–2511. [DOI] [PubMed] [Google Scholar]
- 97. Vlenterie M, Litiere S, Rizzo E, et al. Outcome of chemotherapy in advanced synovial sarcoma patients: review of 15 clinical trials from the European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group; setting a new landmark for studies in this entity. Eur J Cancer 2016; 58: 62–72. [DOI] [PubMed] [Google Scholar]
- 98. Fiore M, Grosso F, Lo Vullo S, et al. Myxoid/round cell and pleomorphic liposarcomas: prognostic factors and survival in a series of patients treated at a single institution. Cancer 2007; 109: 2522–2531. [DOI] [PubMed] [Google Scholar]
- 99. Eilber FC, Eilber FR, Eckardt J, et al. The impact of chemotherapy on the survival of patients with high-grade primary extremity liposarcoma. Ann Surg 2004; 240: 686–695; discussion 95–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Desar IM, Constantinidou A, Kaal SE, et al. Advanced soft-tissue sarcoma and treatment options: critical appraisal of trabectedin. Cancer Manag Res 2016; 8: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Forni C, Minuzzo M, Virdis E, et al. Trabectedin (ET-743) promotes differentiation in myxoid liposarcoma tumors. Mol Cancer Ther 2009; 8: 449–457. [DOI] [PubMed] [Google Scholar]
- 102. Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol 2007; 8: 595–602. [DOI] [PubMed] [Google Scholar]
- 103. Gronchi A, Bui BN, Bonvalot S, et al. Phase II clinical trial of neoadjuvant trabectedin in patients with advanced localized myxoid liposarcoma. Ann Oncol 2012; 23: 771–776. [DOI] [PubMed] [Google Scholar]
- 104. Gronchi A, Strauss DC, Miceli R, et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): a report on 1007 patients from the multi-institutional collaborative RPS working group. Ann Surg 2016; 263: 1002–1009. [DOI] [PubMed] [Google Scholar]
- 105. Crago AM, Socci ND, DeCarolis P, et al. Copy number losses define subgroups of dedifferentiated liposarcoma with poor prognosis and genomic instability. Clin Cancer Res 2012; 18: 1334–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013; 499: 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chabanon RM, Pedrero M, Lefebvre C, et al. Mutational landscape and sensitivity to immune checkpoint blockers. Clin Cancer Res 2016; 22: 4309–4321. [DOI] [PubMed] [Google Scholar]