Abstract
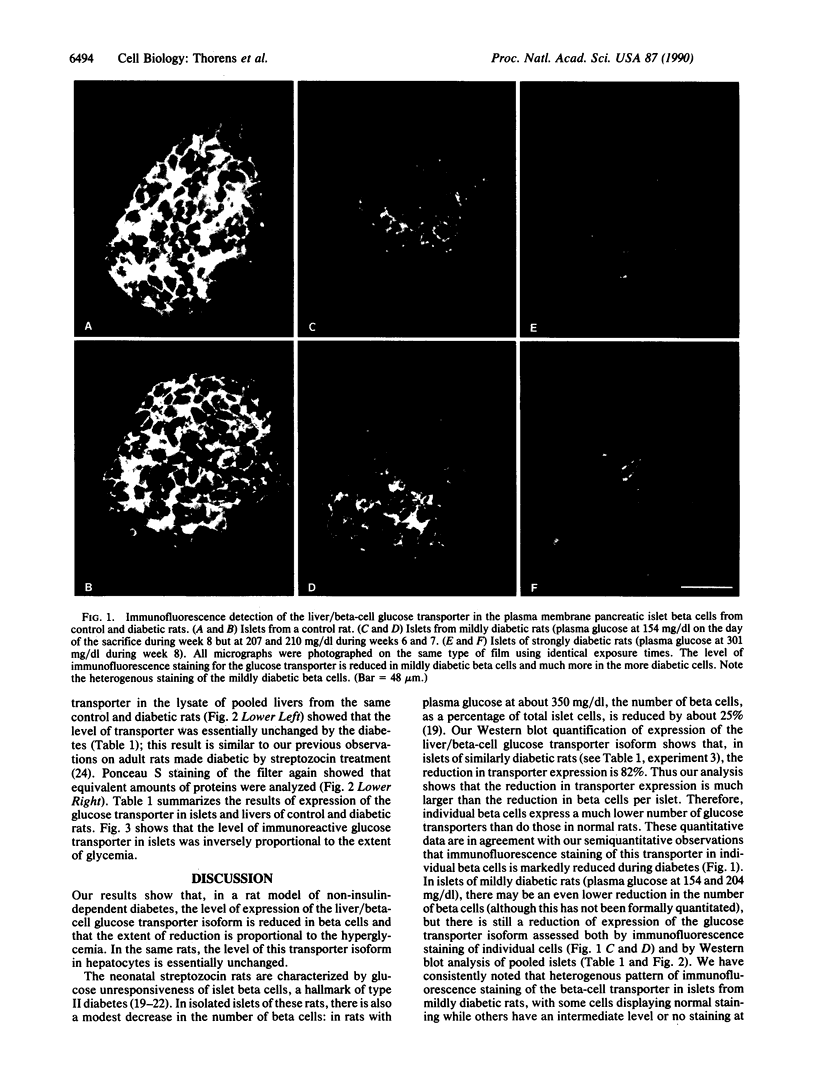
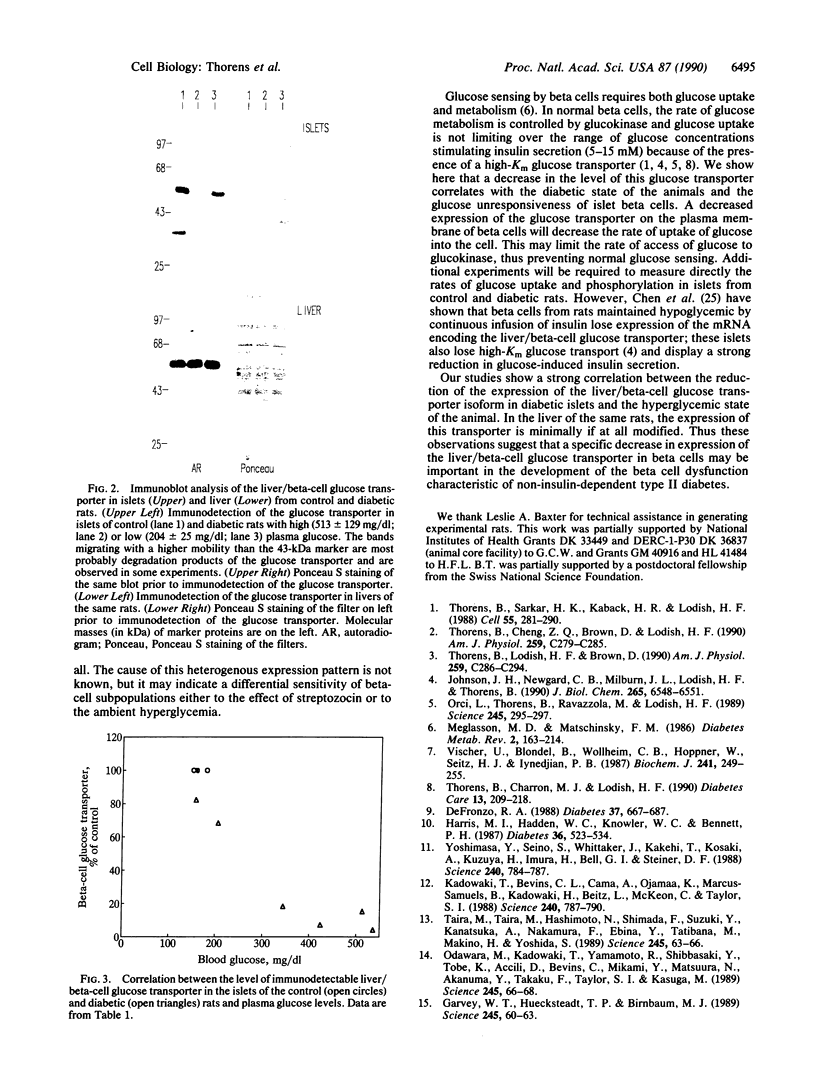
Rats injected with a single dose of streptozocin at 2 days of age develop non-insulin-dependent diabetes 6 weeks later. The pancreatic beta islet cells of these diabetic rats display a loss of glucose-induced insulin secretion while maintaining sensitivity to other secretagogues such as arginine. We analyzed the level of expression of the liver/beta-cell glucose transporter isoform in diabetic islets by immunofluorescence staining of pancreas sections and by Western blotting of islet lysates. Islets from diabetic animals have a reduced expression of this beta-cell-specific glucose transporter isoform and the extent of reduction is correlated with the severity of hyperglycemia. In contrast, expression of this transporter isoform in liver is minimally modified by the diabetes. Thus a decreased expression of the liver/beta-cell glucose transporter isoform in beta cells is associated with the impaired glucose sensing characteristic of diabetic islets; our data suggest that this glucose transporter may be part of the beta-cell glucose sensor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger J., Biswas C., Vicario P. P., Strout H. V., Saperstein R., Pilch P. F. Decreased expression of the insulin-responsive glucose transporter in diabetes and fasting. Nature. 1989 Jul 6;340(6228):70–72. doi: 10.1038/340070a0. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Trent D. F., Honey R. N., Weir G. C. Responses of neonatal rat islets to streptozotocin: limited B-cell regeneration and hyperglycemia. Diabetes. 1981 Jan;30(1):64–69. doi: 10.2337/diab.30.1.64. [DOI] [PubMed] [Google Scholar]
- Chen L., Alam T., Johnson J. H., Hughes S., Newgard C. B., Unger R. H. Regulation of beta-cell glucose transporter gene expression. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4088–4092. doi: 10.1073/pnas.87.11.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988 Jun;37(6):667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Huecksteadt T. P., Birnbaum M. J. Pretranslational suppression of an insulin-responsive glucose transporter in rats with diabetes mellitus. Science. 1989 Jul 7;245(4913):60–63. doi: 10.1126/science.2662408. [DOI] [PubMed] [Google Scholar]
- Giroix M. H., Portha B., Kergoat M., Bailbe D., Picon L. Glucose insensitivity and amino-acid hypersensitivity of insulin release in rats with non-insulin-dependent diabetes. A study with the perfused pancreas. Diabetes. 1983 May;32(5):445–451. doi: 10.2337/diab.32.5.445. [DOI] [PubMed] [Google Scholar]
- Harris M. I., Hadden W. C., Knowler W. C., Bennett P. H. Prevalence of diabetes and impaired glucose tolerance and plasma glucose levels in U.S. population aged 20-74 yr. Diabetes. 1987 Apr;36(4):523–534. doi: 10.2337/diab.36.4.523. [DOI] [PubMed] [Google Scholar]
- Johnson J. H., Newgard C. B., Milburn J. L., Lodish H. F., Thorens B. The high Km glucose transporter of islets of Langerhans is functionally similar to the low affinity transporter of liver and has an identical primary sequence. J Biol Chem. 1990 Apr 25;265(12):6548–6551. [PubMed] [Google Scholar]
- Kadowaki T., Bevins C. L., Cama A., Ojamaa K., Marcus-Samuels B., Kadowaki H., Beitz L., McKeon C., Taylor S. I. Two mutant alleles of the insulin receptor gene in a patient with extreme insulin resistance. Science. 1988 May 6;240(4853):787–790. doi: 10.1126/science.2834824. [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Charron M. J., Lodish H. F., Cushman S. W., Flier J. S. Differential regulation of two glucose transporters in adipose cells from diabetic and insulin-treated diabetic rats. J Clin Invest. 1989 Aug;84(2):404–411. doi: 10.1172/JCI114180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Meglasson M. D., Matschinsky F. M. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2(3-4):163–214. doi: 10.1002/dmr.5610020301. [DOI] [PubMed] [Google Scholar]
- Odawara M., Kadowaki T., Yamamoto R., Shibasaki Y., Tobe K., Accili D., Bevins C., Mikami Y., Matsuura N., Akanuma Y. Human diabetes associated with a mutation in the tyrosine kinase domain of the insulin receptor. Science. 1989 Jul 7;245(4913):66–68. doi: 10.1126/science.2544998. [DOI] [PubMed] [Google Scholar]
- Orci L., Thorens B., Ravazzola M., Lodish H. F. Localization of the pancreatic beta cell glucose transporter to specific plasma membrane domains. Science. 1989 Jul 21;245(4915):295–297. doi: 10.1126/science.2665080. [DOI] [PubMed] [Google Scholar]
- Portha B., Levacher C., Picon L., Rosselin G. Diabetogenic effect of streptozotocin in the rat during the perinatal period. Diabetes. 1974 Nov;23(11):889–895. doi: 10.2337/diab.23.11.889. [DOI] [PubMed] [Google Scholar]
- Sivitz W. I., DeSautel S. L., Kayano T., Bell G. I., Pessin J. E. Regulation of glucose transporter messenger RNA in insulin-deficient states. Nature. 1989 Jul 6;340(6228):72–74. doi: 10.1038/340072a0. [DOI] [PubMed] [Google Scholar]
- Taira M., Taira M., Hashimoto N., Shimada F., Suzuki Y., Kanatsuka A., Nakamura F., Ebina Y., Tatibana M., Makino H. Human diabetes associated with a deletion of the tyrosine kinase domain of the insulin receptor. Science. 1989 Jul 7;245(4913):63–66. doi: 10.1126/science.2544997. [DOI] [PubMed] [Google Scholar]
- Thorens B., Charron M. J., Lodish H. F. Molecular physiology of glucose transporters. Diabetes Care. 1990 Mar;13(3):209–218. doi: 10.2337/diacare.13.3.209. [DOI] [PubMed] [Google Scholar]
- Thorens B., Cheng Z. Q., Brown D., Lodish H. F. Liver glucose transporter: a basolateral protein in hepatocytes and intestine and kidney cells. Am J Physiol. 1990 Dec;259(6 Pt 1):C279–C285. doi: 10.1152/ajpcell.1990.259.2.C279. [DOI] [PubMed] [Google Scholar]
- Thorens B., Flier J. S., Lodish H. F., Kahn B. B. Differential regulation of two glucose transporters in rat liver by fasting and refeeding and by diabetes and insulin treatment. Diabetes. 1990 Jun;39(6):712–719. doi: 10.2337/diab.39.6.712. [DOI] [PubMed] [Google Scholar]
- Thorens B., Lodish H. F., Brown D. Differential localization of two glucose transporter isoforms in rat kidney. Am J Physiol. 1990 Dec;259(6 Pt 1):C286–C294. doi: 10.1152/ajpcell.1990.259.2.C286. [DOI] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Vischer U., Blondel B., Wollheim C. B., Höppner W., Seitz H. J., Iynedjian P. B. Hexokinase isoenzymes of RIN-m5F insulinoma cells. Expression of glucokinase gene in insulin-producing cells. Biochem J. 1987 Jan 1;241(1):249–255. doi: 10.1042/bj2410249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir G. C., Clore E. T., Zmachinski C. J., Bonner-Weir S. Islet secretion in a new experimental model for non-insulin-dependent diabetes. Diabetes. 1981 Jul;30(7):590–595. doi: 10.2337/diab.30.7.590. [DOI] [PubMed] [Google Scholar]
- Yoshimasa Y., Seino S., Whittaker J., Kakehi T., Kosaki A., Kuzuya H., Imura H., Bell G. I., Steiner D. F. Insulin-resistant diabetes due to a point mutation that prevents insulin proreceptor processing. Science. 1988 May 6;240(4853):784–787. doi: 10.1126/science.3283938. [DOI] [PubMed] [Google Scholar]