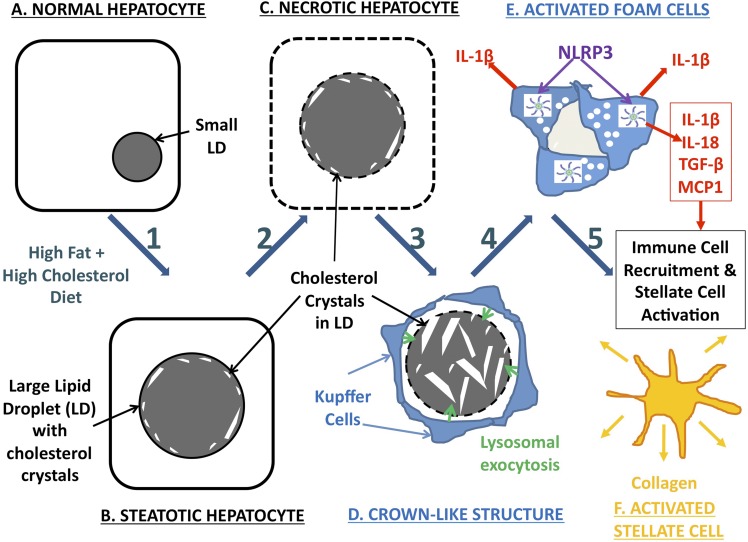
Fig. 7.
Schematic representation of our hypothesized mechanism of hepatic cholesterol crystal–induced NASH, involving the following components: Steps 1–2: As a result of a HFHC diet (A) or other causes of hepatic cholesterol loading, large lipid droplets form within hepatocytes (B), and cholesterol crystallization occurs (C) initially in the periphery of large LDs in close association with the LD membrane, likely as a result of precipitation of supersaturated cholesterol from the LD membrane. Cholesterol crystallization disrupts LD membrane function. Step 3: KCs aggregate around necrotic hepatocytes containing cholesterol crystals in response to the chemotactic signals produced by the hepatocytes, forming CLSs. KCs in the CLS hydrolyze the remnant LDs of dead hepatocytes in the extracellular space, possibly by lysosomal exocytosis (D), and additional cholesterol crystals are formed because of the hydrolysis of cholesterol esters into FC by lysosomal acid lipase. Step 4: Uptake of this FC by KCs and exposure to cholesterol crystals transform KCs into activated lipid-laden foam cells (E). Exposure of KCs to cholesterol crystals causes activation of the NLRP3 inflammasome within KCs, which leads to production of proinflammatory cytokines and chemokines by KCs and propagates the chronic “sterile” inflammation of NASH. Step 5: Chemotactic signals produced by crystal-activated KCs attract an inflammatory infiltrate of additional KCs and neutrophils, as well as causing aggregation, activation, and transformation of stellate cells into collagen-producing myofibroblasts (F), leading to fibrosing NASH and, ultimately, cirrhosis.