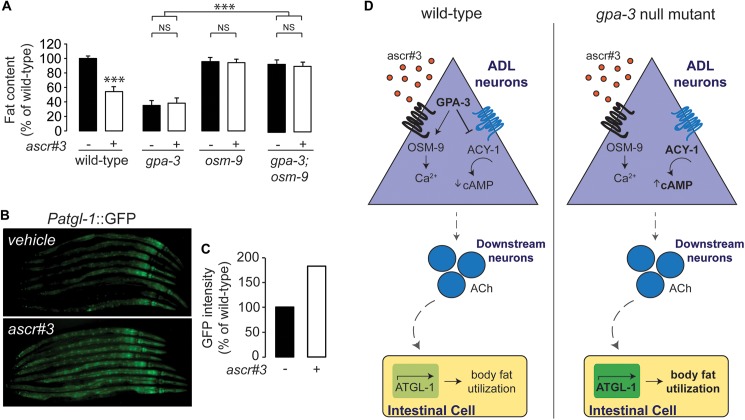
Fig 7. Pheromone signaling regulates body fat stores via GPA-3 signaling.
(A) Animals were transferred at L4 to plates containing either ddH2O vehicle or 80nM ascaroside, ascr#3, then fixed and stained with Oil Red O. Fat content was quantified for each condition and is expressed as a percentage of vehicle-treated wild-type animals ± SEM (n = 20). NS, not significant; ***, p<0.001 by two-way ANOVA. (B) Representative images are shown of wild-type animals bearing an integrated atgl-1::GFP transgene exposed either to ascr#3 or vehicle. (C) The fluorescence intensity of atgl-1 expression was quantified for 8 randomly selected worms for each condition and is expressed as a percentage of wild-type animals exposed to vehicle. (D) Model depicting the ascr#3-mediated regulation of cAMP signaling, via the G protein, GPA-3, in the ADL neurons which controls acetylcholine release in to-be-defined cholinergic neurons, and in turn regulates a stimulatory signal to control body fat stores via the rate-limiting ATGL-1 lipase in the intestine. Under normal conditions, in wild-type animals, environmental levels of the ascaroside ascr#3 regulate the extent to which GPA-3 in the ADL neurons inhibits the downstream adenylyl cyclase ACY-1 and, therefore, controlling levels of cAMP in the ADL neurons. This, in turn, controls the level of acetylcholine released from small, clear vesicles in downstream neurons, and initiates a signal to the intestinal cells to regulate the activity of ATGL-1 (left panel). In gpa-3 mutants, irrespective of ascr#3 levels, the GPA-3 mediated inhibition of ACY-1 is lost, causing more cAMP to be produced in the ADL neurons. This, in turn, causes more acetylcholine to be released in downstream neurons, ultimately up-regulating ATGL-1 in the intestinal cells, and a constitutive loss of body fat due to increased fat utilization (right panel).