Abstract
Crystalline TiO2 has shown its great photocatalytic properties in bacterial inactivation. This work presents a design fabrication of low-cost, layered TiO2 films assembled reactors and a study of their performance for a better understanding to elucidate the photocatalytic effect on inactivation of E. coli in water. The ability to reduce the number of bacteria in water samples for the layered TiO2 composing reactors has been investigated as a function of time, while varying the parameters of light sources, initial concentration of bacteria, and ratios of TiO2 film area and volume of water. Herein, the layered TiO2 films have been fabricated on the glass plates by thermal spray coating prior to screen printing, allowing a good adhesion of the films. Surface topology and crystallographic phase of TiO2 for the screen-printed active layer have been characterized, resulting in the ratio of anatase:rutile being 80:20. Under exposure to sunlight and a given condition employed in this study, the optimized film area:water volume of 1:2.62 has shown a significant ability to reduce the E. coli cells in water samples. The ratio of surface area of photocatalytic active base to volume of water medium is believed to play a predominant role facilitating the cells inactivation. The kinetic rate of inactivation and its behavior are also described in terms of adsorption of reaction species at different contact times.
Keywords: TiO2, solar radiation, inactivation, E. coli, photocatalytic process
1. Introduction
Escherichia coli bacteria found in the contaminated wastewater is known to significantly affect human health. Usually, E. coli inactivation can be done by a variety of methods, such as boiling, solar heating, radiating, filtering with filter paper or sheet, and applying certain chemicals to annihilate the cells of microorganisms [1,2,3,4,5]. Solar irradiation is an effectively convenient method of inactivation. Conroy et al. [6] used batch processing of solar inactivation for improving the quality of biologically contaminated drinking water in developing countries. The technique involved storing the contaminated drinking water in the transparent containers, e.g., plastic bags, plastic bottles, and glass bottles that were placed directly under sunlight for eight hours before consumption. McGuigan et al. [7] has also shown the high effectiveness of irradiation on cell inactivation against a broad range of bacterial pathogens.
Titanium dioxide (TiO2) is known as an extensively used material offering great potential for photocatalytic inactivation in water [8,9,10,11] and treatment of organic contaminants [12,13,14]. Likewise, Nesic et al. found that the E. coli inactivation of TiO2 aggregates in the absence of light [15]. A TiO2 photocatalyst is used to combine with solar irradiation in order to enhance inactivation of bacteria [16,17,18,19]; the use of commercial TiO2 in E. coli inactivation has been widely reported in literature [20,21,22]. In the photocatalytic process, TiO2 is known to generate the reactive oxygen species such as superoxide radical anion (O2•−) and hydroxyl radical (HO•) [23,24] after the generated electron–hole pairs reacted, respectively, with O2 and H2O [25]. The hydroxyl radical species is produced when TiO2 is excited by UV radiation of wavelength near 390 nm [26]. After being exposed to UV light higher than its band gap energy, the anatase form releases the radical species, resulting in oxidative stresses towards microorganisms and thus cell death [27,28,29,30].
The most active crystalline structure of TiO2 for generating free radical species is anatase. Zuccheri et al. reported the use of TiO2 nanoparticles in the formulation of interior paints possessing anti-bacterial activity, as the suitable crystalline structure of TiO2 in the formulation was found to contain 85% of anatase. Duffy et al. [31] has confirmed that the TiO2 coatings can be used to accelerate the inactivation rate of bacterial pathogens, with the addition of TiO2 coated inserts resulting in the improved efficiency of bacterial inactivation by 20%–25%. Recently, the Kiwi group has sputtered TiO2 on polyethylene (PE) fabrics for antibacterial purpose. The work has revealed the necessity of high-anatase TiO2 to induce E. coli inactivation in a minute range under simulated sunlight irradiation, with the higher TiO2 loading leading to faster bacterial inactivation kinetics on the PE surface [32]. For dye degradation purposes, the mixed TiO2 phases with high anatase in the PE-TiO2 sputtered film has been found effective in the discoloration of methylene blue (MB) under solar simulated light [33].
Thermal spraying is a feasible and rapid technique to prepare the coating film on plates from the conventional TiO2 powder [34], allowing for the titania coatings with enhanced mechanical performance. Nararom et al. [35] reported the inactivation of E. coli in water by using solar heating with TiO2 inserted plates, fabricated by thermal spray coating, with an aim to study the photocatalytic process associated with a compound parabolic concentrator by solar heating. The reactor surface area of 0.014 m2 illuminated by the incoming photon with accumulated solar energy of 10 kJ/L was found sufficient to reduce the number of E. coli by two logs colony-forming unit (CFU). However, the continuous flow system of water through the reactor has no significant effect on the improvement of solar inactivation [35].
This work presents a design process of low-cost, layered TiO2 films assembled reactor combining with solar water heating and a study of their performance towards the enhancement of E. coli inactivation. The fabrication of double-type TiO2 film layers consists of thermal spray coating and screen printing layers. To investigate the bactericidal activity of the TiO2 films’ assembled reactors, the effectiveness on the cells’ inactivation is compared in view of different light sources, initial cell concentration, and ratio of the fabricated film area per volume of water. The kinetic behavior of the E. coli inactivation is elucidated for a better understanding by considering the time dependence of E. coli inactivation.
2. Results
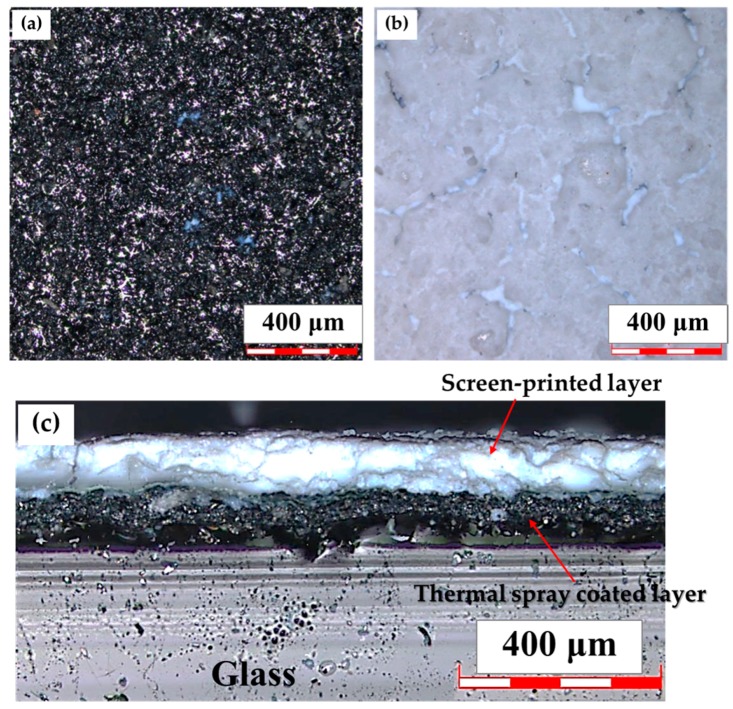
Figure 1a,b show the top surface of the coated films obtained by thermal spray coating and screen printing, and the cross section of those films is shown in Figure 1c. The film thickness achieved by the thermal spray coating and the screen printing is approximately 50 μm and 100 μm, respectively, with the color of the films appearing in dark gray and white. The coated layer was found perfectly adherent to its support—the thermal spray layer on the sand blasted glass surface and the screen-printed film on the thermal spray coated layer. Adhesion of the screen-printed films appeared to improve due to the large groove dimension and a certain degree of surface roughness on the thermal spray layer. Particularly for the screen-printed film, the TiO2 paste formulation and multi-layered film fabrication needed to be developed and seamlessly interfaced towards the improvement of the film adhesion. The mixture of viscous binder and the right amount of TiO2 powder could help regulate the paste viscosity while allowing for the increased porosity to be created in the sintered film, with the pore width of 99–156 Å found to be the most critical film property in terms of mechanical stability and adhesion enhancement to the substrate [36]. The binder is used to create voids between the particles and acts as an important role during sintering as it subsequently affects the film density, the topological structure, and the final strength of the film. A proper film thickness and film stability are strongly required regarding the environmental concern to the release of TiO2 during the wastewater treatment [37,38]. Top view topology of the entire screen-printed film revealed a uniform dispersion of particle agglomerates (see Figure 2b).
Figure 1.
Optical images obtained by confocal microscope showing the (a) top view of thermal spray coated film; (b) top view of screen-printed film; and (c) cross section of the screen-printed layer on the thermal spray coated layer.
Figure 2.
Field emission scanning electron microscopy (FESEM) images of the TiO2 film surface fabricated by (a,b) thermal spray coating and (c,d) screen-printing.
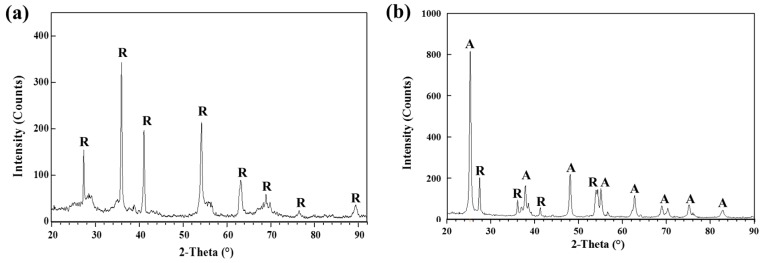
The crystallographic phase of TiO2 coated films by thermal spray coating and screen printing was characterized by X-ray diffraction (XRD) (see Figure 3a,b). The crystallite size of TiO2 particles on the fabricated films was calculated by the Scherrer equation through full width at half maximum (FWHM) of the diffraction peaks of anatase (101) and rutile (110) [39]. The thermal spray coated film resulted solely in the rutile phase, with the calculated crystallite size of 79 nm. However, the screen-printed film has shown the mixed phase of anatase and rutile in a crystalline structure ratio of 80:20, with the calculated crystallite size of 20 nm and 27 nm, respectively.
Figure 3.
X-ray diffraction patterns of the films fabricated by (a) thermal spray coating and (b) screen printing. The marked letters of A and R indicate respectively the TiO2 phases of anatase and rutile.
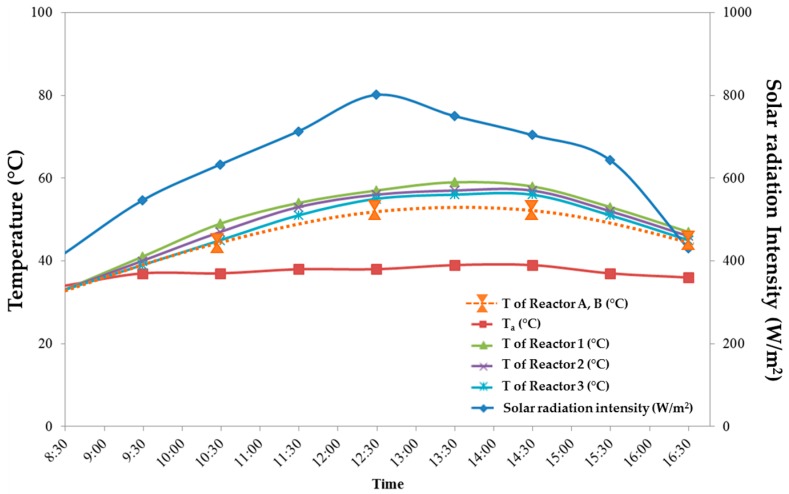
After assembling the glasses with the fabricated films into the reactors, the experiment was performed. Solar radiation intensity and temperatures of all reactors were monitored over the entire period of operation; the results are shown in Figure 4. During the operation, the temperature of water samples in all reactors increased from about 35 °C to its maximum in a window range of 20 °C. The maximum temperature is 53 °C for Reactors A and B, and, respectively, 59, 57, 56 °C for Reactors 1, 2, and 3 after 6 h contact time. The temperature of the inactivation reactors appeared to be in parallel dependent upon the intensity of solar radiation. The maximum solar radiation intensity was found at 12:30 p.m.; 801 W/m2.
Figure 4.
Plots of solar radiation intensity, ambient air temperature (Ta), and reactor temperature during operation time from 08:30 a.m.–4:30 p.m.
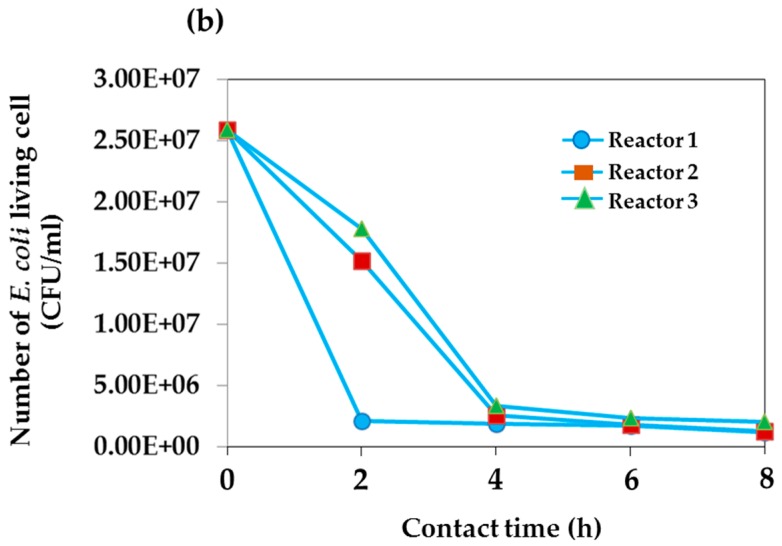
Performance of the TiO2 fabricated film on E. coli inactivation was investigated as a function of contact time. In this study, the experiment was designed to evaluate the bactericidal activity of the TiO2 films assembled reactors and compare their effectiveness in view of different light sources and ratios of the fabricated film area per volume of water on the cells inactivation. The total numbers of living E. coli cells collected at each operation time interval are shown in Table 1; the corresponding results in percentage numbers of living E. coli cell counts over the 8 h of operation are shown in Table 2.
Table 1.
Total numbers of E. coli living cells in the reactors at each operation time interval.
| Time | Total Number of Living E. coil (CFU/mL) | |||||
|---|---|---|---|---|---|---|
| Reactor A 1 (Water + E. coli + Sun) | Reactor B 1,† (Water + E. coli + TiO2 + Sun) | Reactor C 1,† (Water + E. coli + TiO2 + UV) | Reactor 1 † | Reactor 2 † | Reactor 3 † | |
| 8:30 | 2.15 × 1011 | 2.15 × 1011 | 2.15 × 1011 | 2.59 × 107 | 2.59 × 107 | 2.59 × 107 |
| 10:30 | 1.53 × 1011 | 1.10 × 1011 | 8.65 × 1010 | 2.10 × 106 | 1.52 × 107 | 1.78 × 107 |
| 12:30 | 8.55 × 1010 | 7.70 × 1010 | 7.60 × 1010 | 1.85 × 106 | 2.60 × 106 | 3.35 × 106 |
| 14:30 | 8.15 × 1010 | 7.40 × 1010 | 4.65 × 1010 | 1.75 × 106 | 1.80 × 106 | 2.30 × 106 |
| 16:30 | 6.75 × 1010 | 4.45 × 1010 | 3.75 × 1010 | 1.20 × 106 | 1.25 × 106 | 2.05 × 106 |
1 Volume of water in the reactor is 660 cm3; † Area of screen-printed TiO2 film is 0.0252 m2. The ratio of TiO2 film:water volume in Reactors B and C is 1:2.62. Volume of water in Reactors 1, 2, and 3 is 660, 1320, and 1980 cm3; thus, the ratio of TiO2 film:water volume in the reactor is 1:2.62, 1:5.24, 1:7.86, respectively in that order.
Table 2.
Percentage of number of E. coli living cells in the reactors at each operation time interval.
| Contact Time (h) | Percentage of Number of Living E. coli (%) | |||||
|---|---|---|---|---|---|---|
| Reactor A 1 (Water + E. coli + Sun) | Reactor B 1,† (Water + E. coli + TiO2 + Sun) | Reactor C 1,† (Water + E. coli + TiO2 + UV) | Reactor 1 † | Reactor 2 † | Reactor 3 † | |
| 0 | 100 | 100 | 100 | 100 | 100 | 100 |
| 2 | 71.2 | 51.2 | 40.2 | 8.1 | 58.7 | 68.7 |
| 4 | 39.8 | 35.8 | 35.4 | 7.1 | 10.0 | 12.9 |
| 6 | 37.9 | 34.4 | 21.6 | 6.8 | 6.9 | 8.9 |
| 8 | 31.4 | 20.7 | 17.4 | 4.6 | 4.8 | 7.9 |
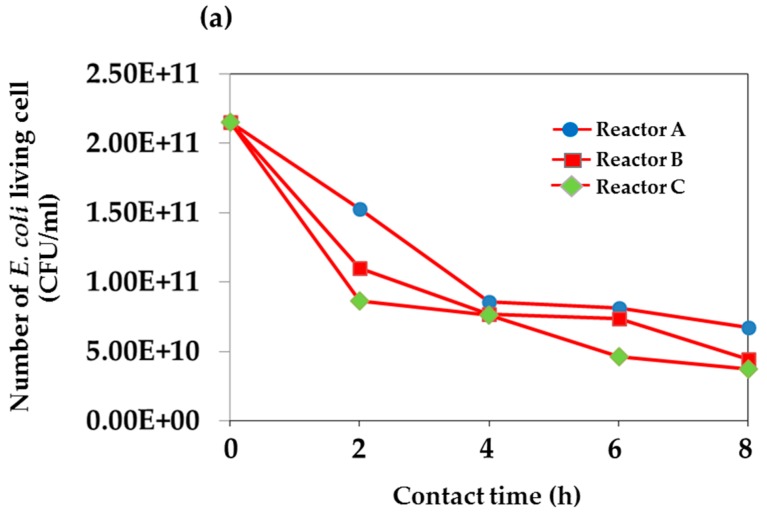
Figure 5a shows the plots of total numbers of E. coli living cells against contact time for Reactors A, B, and C. Using the E. coli initial concentration of 2.15 × 1011 CFU/mL, the effect of UV light for Reactor C has undoubtedly shown its more significant effect on the inactivation of the E. coli cells than that of the sunlight, Reactor B. The percentage reduction of E. coli determined for the UV light and the sunlight conditions are, respectively, 59.8% and 48.8%. The explanation regarding the photocatalytic inactivation ability of TiO2 has been described in many reports [40,41,42]. With relatively low energy of UV light, the cells could be damaged through the oxidation stress caused by the oxygen radicals within the cells [43]. The reactor without the fabricated TiO2 film showed the lowest percentage reduction, 28.8%, of E. coli after the 2 h contact time.
Figure 5.
Plots of total number of E. coli living cells (CFU/mL) and contact time (h) obtained from the test of reactor under exposure to sunlight over 8 h operation period for (a) Reactors A, B, and C; and (b) Reactors 1, 2, and 3.
The initial concentration was adjusted to the range of 2.59 × 107 CFU/mL and the dilution effect was found to enhance the E. coli inactivation ability. Figure 5b demonstrates the plots of total numbers of E. coli living cells against time as a function of water volume used in Reactors 1, 2, and 3; i.e., 660 cm3, 1320 cm3, and 1980 cm3, respectively. At the beginning of the experiment, the initial quantity of total living E. coli in Reactors 1–3 was fixed at the same concentration of 2.59 × 107 CFU/mL or 100%. The curve for Reactor 1 appeared to drastically decrease within the first 2 h, compared to the gradual decrease over the 4 h contact time for Reactors 2 and 3. For a given condition employed in this study, the percentage of numbers of living E. coli cells was found to be 8.1% for the area of screen-printed TiO2 film:volume of water in the reactor was 1:2.62. After increasing the water volume in the ratio by a factor of 2 or 3, the E. coli reduction efficiency did not improve, as seen for Reactors 2 and 3. After 4 h, all curves slowly declined until the end of the testing period. For the first 2 h of contact time, the surface area of photocatalytic active species is believed to play a predominant role affecting the cells’ inactivation, as water volume is relatively smaller. As a consequence, there would be an increasing degree of adsorption and hence inactivation ability due to the greater availability of bacteria on the photocatalyst surface [17]. In this case, the combined effect of water temperature in the reactors of approximately less than 50 °C was considered to be insignificant. During this first operation period, the temperature of water sample in Reactors 1–3 was less than 50 °C. It was particularly noted in the literature that the water temperature up to 45 °C has no effect on the inactivation of E. coli, as the temperature higher than 50 °C due to radiation could promote the pasteurizing effect on the bacteria cells [44].
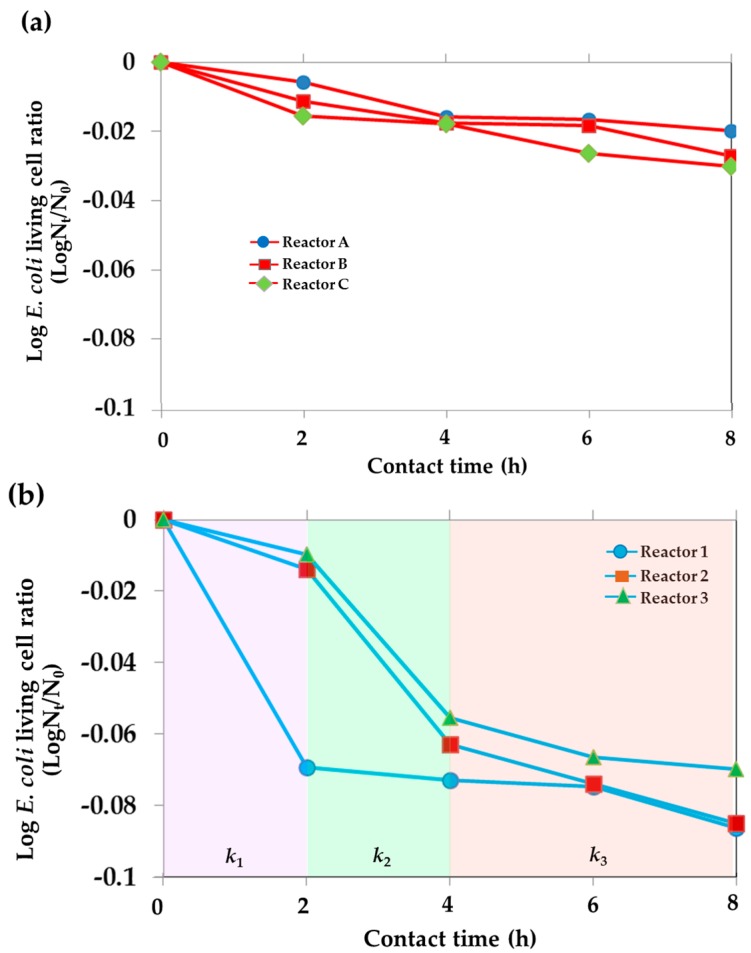
In this study, the efficiency of the reactors on reducing the E. coli cells as a function of contact time was evaluated in terms of kinetic rate, as the plots shown in Figure 6. Figure 6a appears to show a linear dependence of E. coli inactivation ability upon contact time over the 8 h duration. For Reactors A, B, and C, the inactivation follows the first order dependence (Log10(Nt/N0) = −kt, where Nt = total number of E. coli living cells at time t, N0 = total number of E. coli living cells at time 0, and k = rate constant). As presented in Table 3, it is apparent that kReactor C > kReactor B > kReactor A, confirming the photocatalytic inactivation of the TiO2 film that is considerably sensitive to UV during 8 h of operation.
Figure 6.
Kinetic data plots of E. coli inactivation for (a) Reactors A, B, and C and (b) Reactors 1, 2, and 3.
Table 3.
Rate constant determined from slope of the plots in Figure 6a,b at each operation period.
| Condition | Rate Constant (k)[Contact Time Duration] | ||
|---|---|---|---|
| Reactor A (Water + E. coli + Sun) | 0.0050[2–8 h] | ||
| Reactor B (Water + E. coli + TiO2 + Sun) | 0.0061[2–8 h] | ||
| Reactor C (Water + E. coli + TiO2 + UV) | 0.0071[2–8 h] | ||
| k1[0–2 h] | k2[2–4 h] | k3[4–8 h] | |
| Reactor 1 (TiO2 film area:water volume = 1:2.62) | 0.0691 | 0.0053[2–8 h] | |
| Reactor 2 (TiO2 film area:water volume = 1:5.24) | 0.0138 | 0.0490 | 0.0110 |
| Reactor 3 (TiO2 film area:water volume = 1:7.86) | 0.0096 | 0.0458 | 0.0072 |
The curves in Figure 6b for Reactors 1, 2, and 3 also show their behavior in time dependence of cell inactivation, but not in such linear behavior; thus, different k values were examined. During 0–2 h, the rate k1 of Reactor 1 is higher than that of Reactors 2 and 3; i.e., 0.0691, 0.0138, and 0.0096. Thereafter, from 2 h to 8 h, the behavior of the rest of the number of living E. coli cells in the reactor declining directly with the operation time follows the first order dependence. The inactivation ability was found to decrease to about ten times lower; the rate k2 is 0.0053. Reducing the initial concentration of E. coli by four log10 stages in water samples, the inactivation performance of Reactor 1 has improved about ten times higher for the rate of Reactor 1 with respect to Reactor B, under exposure to sunlight. It could be presumed that the diluted concentration of E. coli cells in the water sample has become a facilitating effect on inhibiting the E. coli number.
On further increase in water volume, in turn, the slower rate of E. coli inactivation is obtained. For Reactors 2 and 3, three different rates of inactivation are clearly observed. The rate k2 is largest during 2–4 h of operation, probably due to the greater adsorption of reaction partners to the TiO2 surface, leading to increased effectiveness of the catalysis and thus the enhancement of cell inactivation [45]. During 0–2 h, the adsorption is probably limited by the key factor of water volume determining the mobility of the reaction species to the catalytic surface. For the 2–4 h period, a certain amount of the cells adsorbing on the surface could possibly alter the surface property of the catalytic film. That is, the total surface charge of TiO2 has been altered, to some extent, to become more hydrophobic [46]. As a result, such phenomena could probably induce more adsorption of different charge from water medium, subsequently establishing a certain mass movement of water towards the adsorbate. This hereby could lead to more adsorption of the bacteria on the surface as dependent upon the contact time. The mechanism of mass movement inducing adsorption due to the alternation of surface charge is believed to be an important factor facilitating the cells’ inactivation ability, whereas the reaction rate k3 is prone to linear behavior, as the cells inactivation ability also depends on the concentration of living cells left after largely being inhibited in the first four hours.
3. Materials and Methods
3.1. Film Preparation and Characterization
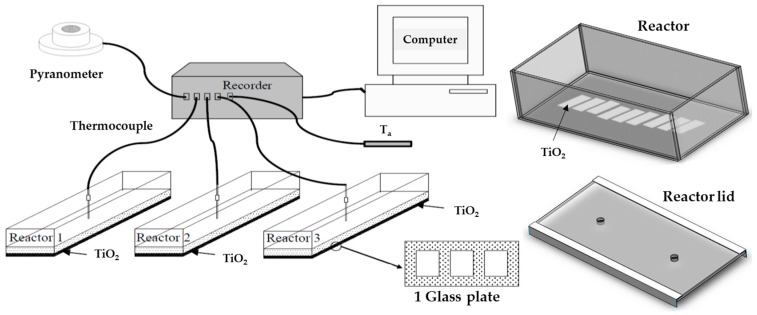
The layered TiO2 films were prepared on glass plates 10 cm × 15 cm in size and were cleaned with soap, rinsed with iso-propanol, and dried in an oven. The entire surface of the glass plates was prepared by sand blasting and thermal spray coated with TiO2 (Amperit® Flame and Plasma Spray Powders, H. C. Starck GmbH, Munich, Germany) and plasma spray powder No. 782.2, 95% of 90 μm size range). The feed rate of thermal spray coating was held fixed at 53 g/min in H2 and Ar air flow, and the thickness was controlled at 50 μm. The second layer of TiO2 film was casted by a screen printing technique on top of the thermal spray coated film. The TiO2 paste was prepared by mixing 20% wt TiO2 (P25 Degussa, Evonik, Hanau, Germany) with 5% Poly (vinyl alcohol) (PVA) in de-ionized water, and coated on the marked area on the thermal spray coated film. In each glass plate, there are three sectional areas of 8 × 3.5 cm2 for screen printing (see the drawing of 1 glass plate in Figure 7). The total area of the screen-printed film per glass plate is 84 cm2. For each glass plate, five replicate layers were done in order to achieve thickness of about 100 μm, as each layer of screen printing was subject to oven drying at 100 °C for 1 h prior to applying its new layer. The annealing process of the screen-printed films was done at 350 °C for 3 h with the ramping rate of 1 °C/min. The cross-sectional view of the TiO2 thermal spray coated film was characterized by confocal microscope (Confocal Olympus OLS 400, Tokyo, Japan) as the thickness was also measured. The phase of the TiO2 screen-printed film was identified by X-ray diffraction (X’Pert PRO PANalytical, PANalytical BV, Almelo, The Netherlands).
Figure 7.
Schematic drawing illustrating connections of the experimental devices employed in the study of inactivation of bacteria.
3.2. Inactivation Reactor
The inactivation reactor was designed to be composed of a base and a cover. The base was made of a box of 3 mm glass in a volume of 15 × 30 × 10 cm3, inserted with three glass plates into the inactivation unit; thus, the total area of the TiO2 screen-printed film per reactor is 252 cm2. The lid of the reactor is a transparent glass sheet, with two holes pierced for putting thermocouples and collecting water from the reactor. The experiment was conducted under sunlight, as intensity of solar radiation was measured by pyranometer (KIPP & ZONEN, CM11, Delft, The Netherlands) and recorded through a Wisco’s Analog Input Module (model AI210, Bangkok, Thailand), while the temperature was also recorded simultaneously. The schematic diagram of the experimental device is illustrated in Figure 7. In this study, there were 6 types of reactors set in different conditions to obtain a better insight into the efficiency of each reactor under various fabrication/test conditions on the bactericidal ability. The conditions for Reactors A, B, C, 1, 2, and 3 are as follows: Reactor A—660 cm3 water volume with E. coli and exposed to sunlight; Reactor B—660 cm3 water volume with E. coli, 0.0252 m2 area of screen-printed TiO2 with 1:2.62 of TiO2 film:water volume ratio, and exposed to sunlight; Reactor C—660 cm3 water volume with E. coli, 0.0252 m2 area of screen-printed TiO2 with 1:2.62 of TiO2 film:water volume ratio, and exposed to UV light; Reactor 1—660 cm3 water volume with E. coli and 1:2.62 of TiO2 film:water volume ratio, and exposed to sunlight; Reactor 2—1320 cm3 water volume with E. coli and 1:5.24 of TiO2 film:water volume ratio, and exposed to sunlight; Reactor 3—1980 cm3 water volume with E. coli and 1:7.86 of TiO2 film:water volume ratio, and exposed to sunlight.
3.3. Unit Testing
The unit was tested under sunlight and UV light for a set period of time; the operation period started from 08:30 a.m. to 16:30 p.m. UV light source was a 125 W lamp with a 0.52 filter. The experiment was designed to study the inactivation efficacy of the reactor, with no flow of water, as the volume of water was varied; 660 cm3, 1320 cm3, and 1980 cm3 designated for Reactor 1, Reactor 2, and Reactor 3, respectively. A tap water sample was boiled and cooled down to room temperature. Escherichia coli (ATCC® 25922™) was cultured in Nutrient Broth (NB) for 24 h and stored in the refrigerator at 4 °C. The required E. coli was adjusted by dilution. The initial concentration was prepared at 107 and 1011 CFU/mL.
3.4. Sample Collection
Water from the reactors was sampled at two-hour intervals during the operation for counting the total number of E. coli living cells; 10 mL of water was collected into sterilized tubes. The samples were then tested with eosin methylene blue (EMB) by incubating at 30 °C for 24 h; and the total number of E. coli living cells was counted. The percentage reduction of E. coli was calculated through the following expression; % reduction = (a − b) × 100/a, where a is number of viable cells (CFU/mL) in the control, and b is number of viable cells (CFU/mL) in the sample.
4. Conclusions
The efficiently photocatalytic performance of layered TiO2 films on treating E. coli cells was investigated. The double-layered TiO2 films with good adhesion were successfully produced by thermal spray coating and screen printing. The film fabrication was manipulated to achieve the total film thickness of approximately 150 µm. The crystalline structure ratio of anatase:rutile for the screen-printed film was characterized as 80:20, as the calculated crystallite size of rutile is larger than that of anatase. The experiment was conducted using two sets of initial E. coli concentrations of 2.15 × 1011 and 2.59 × 107 CFU/mL. The optimized film area:water volume of 1:2.62 has shown a significant ability to reduce the E. coli cells in water samples to 8.1%, under the exposure to sunlight and a given condition employed in this study. The kinetic response of the reactors to the E. coli cells was found to be a linear dependence upon the contact time for the reactors under exposure to UV and sunlight. The mechanism of mass movement inducing adsorption due to the alternation of surface charge was proposed as an important factor facilitating the cells inactivation ability. This work has shown the use of TiO2 P25 as a catalyst in a merit of a design fabrication of low-cost, layered TiO2 film assembled reactors with their potential in E. coli cell inactivation. To some extent, this aspect of work could pave the way towards a consideration of scalable reactors as a possibly complementary treatment process.
Acknowledgments
The authors acknowledge the National Metal and Materials Technology Center (MTEC), Thailand for instrument facilities and providing research funding with Grant No. P1300159 through the Ceramics Technology Research Unit. The authors would also like to thank the School of Energy Environment and Materials, King Mongkut’s University of Technology Thonburi, the College of Engineering, Rangsit University Thailand for supporting the experimental site, and the Faculty of Biotechnology, Rangsit University, Thailand for their help with bacterial concentration analysis.
Author Contributions
Sorachon Yoriya and Wreerat Laithong conceived and designed the experiments; Wreerat Laithong performed the experiments and Angkana Chumphu performed the thickness measurement and XRD characterization; Sorachon Yoriya and Wreerat Laithong analyzed the data; Pusit Pookmanee, Sirichai Thepa, and Roongrojana Songprakorp contributed suggestion; Sorachon Yoriya and Wreerat Laithong wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mcloughlin O.A., Fernandez-Ibanez P., Gernjak W., Rodriguez S.M., Gill L.W. Photocatalytic disinfection of water using low cost compound parabolic collector. Sol. Energy. 2004;77:625–633. doi: 10.1016/j.solener.2004.05.017. [DOI] [Google Scholar]
- 2.Gomes A.I., Santos J.C., Vilar V.J.P., Boaventura R.A.R. Inactivation of bacteria E. coli and photodegradation of humic acids using natural sunlight. Appl. Catal. B. 2009;88:283–291. doi: 10.1016/j.apcatb.2008.11.014. [DOI] [Google Scholar]
- 3.Lonnen J., Kilvington S., Kehoe S.C., Al-Touati F., Mcguigan K.G. Solar and photocatalytic disinfection of protozoan, fungal and bacterial microbes in drinking water. Water Res. 2005;39:877–883. doi: 10.1016/j.watres.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Marques A.R., Gomes F.C.O., Fonseca M.P.P., Parreira J.S., Santos V.P. Efficiency of PET reactors in solar water disinfection for use in southeastern Brazil. Sol. Energy. 2013;87:158–167. doi: 10.1016/j.solener.2012.10.016. [DOI] [Google Scholar]
- 5.Lopez M.I.P., Fernandez-Ibanez P., Ubomba-Jaswa E., Navntoft C., Garcia-Fernandez I., Dunlop P.S.M., Schmid M., Byrne J.A., Mcguigan K.G. Elimination of water pathogens with solar radiation using an automated sequential batch CPC reactor. J. Hazard. Mater. 2011;196:16–21. doi: 10.1016/j.jhazmat.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 6.Conroy R.M., Elmore-Meegan M., Joyce T., McGuigan K.G., Barnes J. Solar disinfection of drinking water and diarrhoea in Maasai children: A controlled field trial. Lancet. 1996;348:1695–1697. doi: 10.1016/S0140-6736(96)02309-4. [DOI] [PubMed] [Google Scholar]
- 7.McGuigan K.G., Joyce T.N., Conroy R.M. Solar disinfection: Use of sunlight to decontaminate drinking water in developing countries. J. Med. Microbiol. 1999;48:785–787. doi: 10.1099/00222615-48-9-785. [DOI] [PubMed] [Google Scholar]
- 8.Al Momani F.A., Shawaqfeh A.T., Shawaqfeh M.A.S. Solar wastewater treatment plant for aqueous solution of pesticide. Sol. Energy. 2007;81:1213–1218. doi: 10.1016/j.solener.2007.01.007. [DOI] [Google Scholar]
- 9.Jung J., Kim J.O., Choi J.Y. Effect of various oxidants in a photocataysis/filtration system for the treatment of contaminants. Res. Chem. Intermed. 2009;35:243–248. doi: 10.1007/s11164-009-0034-8. [DOI] [Google Scholar]
- 10.Paleologou A., Marakas H., Xekoukoulotakis N.P., Moya A., Vergara Y., Kalogerakis N., Gikas P., Mantzavinos D. Disinfection of water and wastewater by TiO2 Photocatalysis, sonolysis and UV-C irradiation. Catal. Today. 2007;129:136–142. doi: 10.1016/j.cattod.2007.06.059. [DOI] [Google Scholar]
- 11.Jain A., Vaya D., Sharma V.K., Ameta S.C. Photo-fenton degradation of phenol red catalyzed by inorganic additives: A technique for wastewater treatment. Kinet. Catal. 2011;52:40–47. doi: 10.1134/S0023158411010071. [DOI] [Google Scholar]
- 12.McCullagh C., Robertson J.M.C., Bahnemann D.W., Robertson P.K.J. The application of TiO2 photocatalysis for disinfection of water contaminated with pathogenic micro-organisms: A review. Res. Chem. Intermed. 2007;33:359–375. doi: 10.1163/156856707779238775. [DOI] [Google Scholar]
- 13.Vorontsov A.V., Kozlova E.A., Besove A.S., Kozlov D.V., Kiselev S.A., Safatov A.S. Photocatalysis: Light energy conversion for the oxidation, disinfection, and decomposition of water. Kinet. Catal. 2010;51:801–808. doi: 10.1134/S0023158410060042. [DOI] [Google Scholar]
- 14.Elfeky S.A., Al-Sherbini A.S.A. Photocatalytic decomposition of trypan blue over nanocomposite thin films. Kinet. Catal. 2011;52:391–396. doi: 10.1134/S0023158411030037. [DOI] [Google Scholar]
- 15.Nesic J., Rtimi S., Laub D., Roglic G.M., Pulgarin C., Kiwi J. New evidence for TiO2 uniform surfaces leading to complete bacterial reduction in the dark: Critical issues. Coll. Surf. B Biointerfaces. 2014;123:593–599. doi: 10.1016/j.colsurfb.2014.09.060. [DOI] [PubMed] [Google Scholar]
- 16.Benabbou A.K., Derriche Z., Felix C., Lejeune P., Guillard C. Photocatalytic inactivation of Escherichia coli-effect of concentration of TiO2 and microorganism, nature, and intensity of UV irradiation. Appl. Catal. B. 2007;76:257–263. doi: 10.1016/j.apcatb.2007.05.026. [DOI] [Google Scholar]
- 17.Malato S., Fernandez-Ibanez P., Maldonado M.I., Blanco J., Gernjak W. Dexontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today. 2009;147:1–59. doi: 10.1016/j.cattod.2009.06.018. [DOI] [Google Scholar]
- 18.Robertson J.M.C., Robertson P.K.J., Lawton L.A. A comparison of the effectiveness of TiO2 photocatalysis and UVA photolysis for the destruction of three pathogenic micro-organisms. J. Photochem. Photobiol. A Chem. 2005;175:51–56. doi: 10.1016/j.jphotochem.2005.04.033. [DOI] [Google Scholar]
- 19.Sichel C., Cara M.D., Tello J., Blanco J., Fernandez-Ibanez P. Solar photocatalytic disinfection of agricultural pathogenic fungi: Fusarium species. Appl. Catal. B Environ. 2007;74:152–160. doi: 10.1016/j.apcatb.2007.02.005. [DOI] [Google Scholar]
- 20.Dunlop P.S.M., Ciavola M., Rizzo L., Byrne J.A. Inactivation and injury assessment of Eschericia coli during solar and photocatalytic disinfection in LDPE bags. Chemosphere. 2011;85:1160–1166. doi: 10.1016/j.chemosphere.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Rincon A.G., Pulgarin C. Photocatalytical inactivation of E. coli: Effect of (continuous-intermittent) light intensity and of (suspended-fixed) TiO2 concentration. Appl. Catal. B Environ. 2003;44:263–284. doi: 10.1016/S0926-3373(03)00076-6. [DOI] [Google Scholar]
- 22.Grieken R.V., Marugan J., Pablos C., Furones L., Lopez A. Comparison between the photocatalytic inactivation of Gram-positive E. faecalis and Gram-negative E. coli faecal contamination indicator microorganism. Appl. Catal. B Environ. 2010;100:212–220. doi: 10.1016/j.apcatb.2010.07.034. [DOI] [Google Scholar]
- 23.Chong M.N., Jin B., Chow C.W., Saint C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010;44:2997–3027. doi: 10.1016/j.watres.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 24.Nikazara M., Gholivand K., Mahanpoor K. Using TiO2 supported on clinoptilolite as a catalyst for photocatalytic degradation of azo dye disperse yellow 23 in water. Kinet. Catal. 2007;48:214–220. doi: 10.1134/S002315840702005X. [DOI] [Google Scholar]
- 25.Fernandez P., Blanco J., Sichel C., Malato S. Water disinfection by solar photocatalysis using compound parabolic collectors. Catal. Today. 2005;101:345–352. doi: 10.1016/j.cattod.2005.03.062. [DOI] [Google Scholar]
- 26.McLoughlin O.A., Kehoe S.C., McGuigan K.G., Duffy E.F., Touati F.A., Gernja W., Alberola I.O., Rodriguez S.M., Gill L.W. Solar disinfection of contaminated water: A comparison of three small-scale reactors. Sol. Energy. 2004;77:657–664. doi: 10.1016/j.solener.2004.07.004. [DOI] [Google Scholar]
- 27.Gaya U.I., Abdullah A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide. J. Photochem. Photobiol. C Photochem. Rev. 2008;9:1–12. doi: 10.1016/j.jphotochemrev.2007.12.003. [DOI] [Google Scholar]
- 28.Desai V.S., Kowshik M. Antimicrobial activity of titanium dioxide nanoparticles synthesized by sol-gel technique. Res. J. Microbiol. 2009;4:97–103. [Google Scholar]
- 29.Chung C.J., Lin H.I., Chou C.M., Hsieh P.Y., Hsiao C.H., Shi Z.Y., He J.L. Inactivation of Staphylococcus aureus and Escherichia coli under various light sources on photocatalytic titanium dioxide thin film. Surf. Coat. Technol. 2009;203:1081–1085. doi: 10.1016/j.surfcoat.2008.09.036. [DOI] [Google Scholar]
- 30.Li Y., Sun X., Li H., Wang S., Wei Y. Preparation of anatase of TiO2 nanoparticles with high thermal stability and specific surface area by alcohothermal method. Powder Technol. 2009;194:149–152. doi: 10.1016/j.powtec.2009.03.041. [DOI] [Google Scholar]
- 31.Duffy E.F., Touati F.A., Kehoe S.C., McLoughlin O.A., Gill L.W., Gernjak W., Oller I., Maldonado M.I., Malato S., Cassidy J., et al. A novel TiO2-assisted solar photocatalytic batch-process disinfection reactor for the treatment of biological and chemical contaminants in domestic drinking water in developing countries. Sol. Energy. 2004;77:649–655. doi: 10.1016/j.solener.2004.05.006. [DOI] [Google Scholar]
- 32.Rtimi S., Sanjines R., Andrzejczuk M., Pulagrin C., Kulik A., Kiwi J. Innovative transparent non-scattering TiO2 bactericide thin films inducing increased E. coli wall fluidity. Surf. Coat. Technol. 2014;254:333–343. doi: 10.1016/j.surfcoat.2014.06.035. [DOI] [Google Scholar]
- 33.Rtimi S., Pulgarin C., Sanjines R., Kiwi J. Kinetics and mechanism for transparent polyethylene-TiO2 films mediated self-cleaning leading to MB dye discoloration under sunlight irradiation. Appl. Catal. B Environ. 2015;162:236–244. doi: 10.1016/j.apcatb.2014.05.039. [DOI] [Google Scholar]
- 34.Ibrahim A., Lima R., Berndt C.C., Marple B. Fatigue and mechanical properties of nanostructured and conventional titania (TiO2) thermal spray coatings. Surf. Coat. Technol. 2007;201:7589–7596. doi: 10.1016/j.surfcoat.2007.02.025. [DOI] [Google Scholar]
- 35.Nararom M., Thepa S., Kongkiattikajorn J., Songprakorp R. The effect of bacterial disinfection by solar illumination and photocatalytic disinfection. Chem. Intermed. 2015;41:6543–6558. doi: 10.1007/s11164-014-1760-0. [DOI] [Google Scholar]
- 36.Yasin A., Guo F., Demopoulos G.P. Aqueous, screen-printable paste for fabrication of mesoporous composite anatase-rutile TiO2 nanoparticle thin films for (Photo)electrochemical devices. ACS Sustain. Chem. Eng. 2016;4:2173–2181. doi: 10.1021/acssuschemeng.5b01625. [DOI] [Google Scholar]
- 37.Dhungel S.K., Park J.G. Optimization of paste formulation for TiO2 nanoparticles with wide range of size distribution for its application in dye sensitized solar cells. Renew. Energy. 2010;35:2776–2780. doi: 10.1016/j.renene.2010.04.031. [DOI] [Google Scholar]
- 38.Lee K.M., Suryanarayanan V., Ho K.C. The influence of surface morphology of TiO2 coating on the performance of dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells. 2006;90:2398–2404. doi: 10.1016/j.solmat.2006.03.034. [DOI] [Google Scholar]
- 39.Klug H.P., Alexander L.E. X-ray Diffraction Procedures: For Polycrystalline and Amorphous Materials. Wiley; New York, NY, USA: 1954. [Google Scholar]
- 40.Matsunaga T., Tomoda R., Nakajima T., Wake H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985;29:211–214. doi: 10.1111/j.1574-6968.1985.tb00864.x. [DOI] [Google Scholar]
- 41.Saito T., Iwase T., Horie J., Morioka T. Mode of photocatalytic bactericidal action of powdered semiconductor TiO2 on mutans streptococci. J. Photochem. Photobiol. B. 1992;14:369–379. doi: 10.1016/1011-1344(92)85115-B. [DOI] [PubMed] [Google Scholar]
- 42.Watts R.J., Kong S., Orr M.P., Miller G.C., Henry B.E. Photocatalytic inactivation of coliform bacteria and viruses in secondary wastewater effluent. Water Res. 1995;29:95–100. doi: 10.1016/0043-1354(94)E0122-M. [DOI] [Google Scholar]
- 43.Bock C., Dittmar H., Gemeinhardt H., Bauer E., Greulich K.O. Comet assay detects cold repair of UV-A damages in human B-lymphoblast cell line. Mutat. Res. 1998;408:111–120. doi: 10.1016/S0921-8777(98)00023-8. [DOI] [PubMed] [Google Scholar]
- 44.McGuigan K.G., Joyce T.M., Conroy R.M., Gillespie J.B., Elmore-Meegan M. Solar disinfection of drinking water contained in transparent plastic bottles: Characterizing the bacterial inactivation process. J. Appl. Microbiol. 1998;84:1138–1148. doi: 10.1046/j.1365-2672.1998.00455.x. [DOI] [PubMed] [Google Scholar]
- 45.Kormann C., Bahnemann D.W., Hoffmann M.R. Photolysis of chloroform and other organic molecules in aqueous TiO2 suspensions. Environ. Sci. Technol. 1991;25:494–500. doi: 10.1021/es00015a018. [DOI] [Google Scholar]
- 46.Gilbert P., Evans D.J., Evans E., Duguld I.G., Brown M.R.W. Surface characteristics and adhesion of E. coli and S. epidermis. J. Appl. Bacteriol. 1991;71:72–77. [PubMed] [Google Scholar]