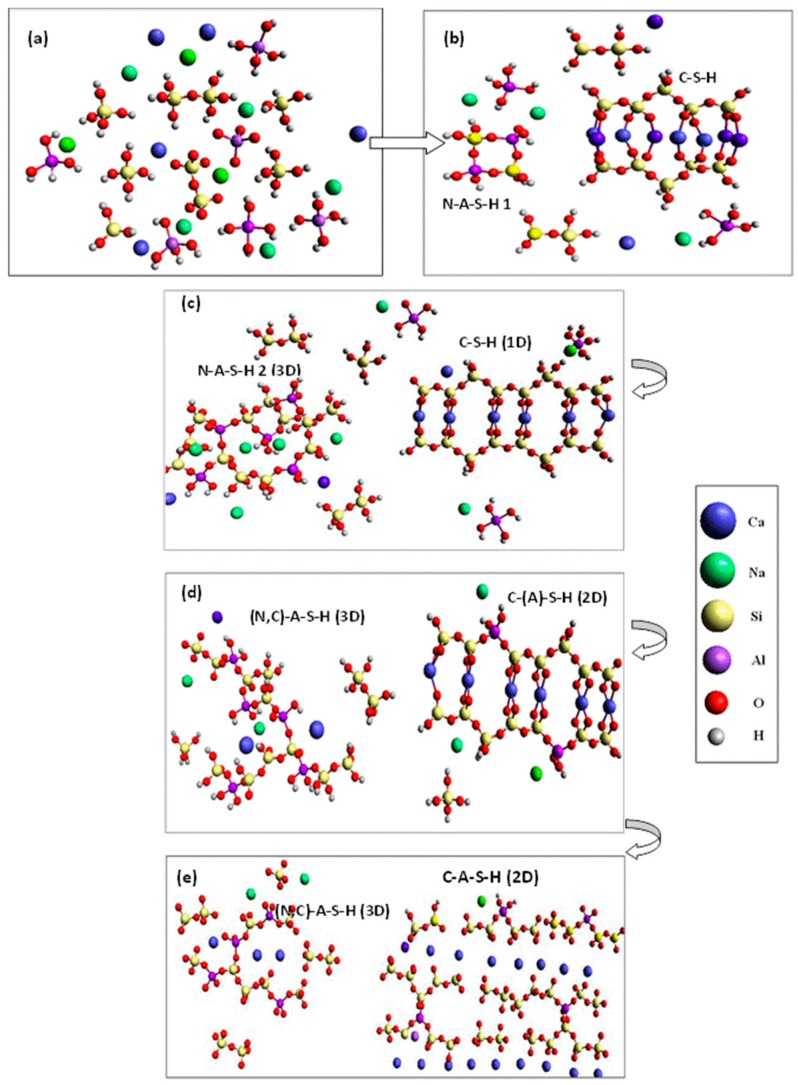
Figure 6.
Nano-structural mechanism for gel formation in hybrid alkaline cements; (a) dissolution of ionic species from the source of alumino-and calcium silicates; (b) precipitation of aluminum-high (type I) N–A–S–H gels and C–S–H gels; (c) silica uptake by both gels with an increase in C–S–H gel mean chain length and the generation of silica-high type 2 N–A–S–H gels; (d) diffusion of aluminum and calcium in the matrix and their uptake, respectively, in C–S–H and N–A–S–H gels to form (N,C)–A–S–H gels; (e) distortion of the (N,C)–A–S–H gel due to the polarizing effect of calcium, leading to its rupture, while the C–A–S–H gel continues to take up aluminum species in bridging positions, favoring chain cross-linking and hence a more polymerized structure (hydrogen bonds omitted in this final stage).