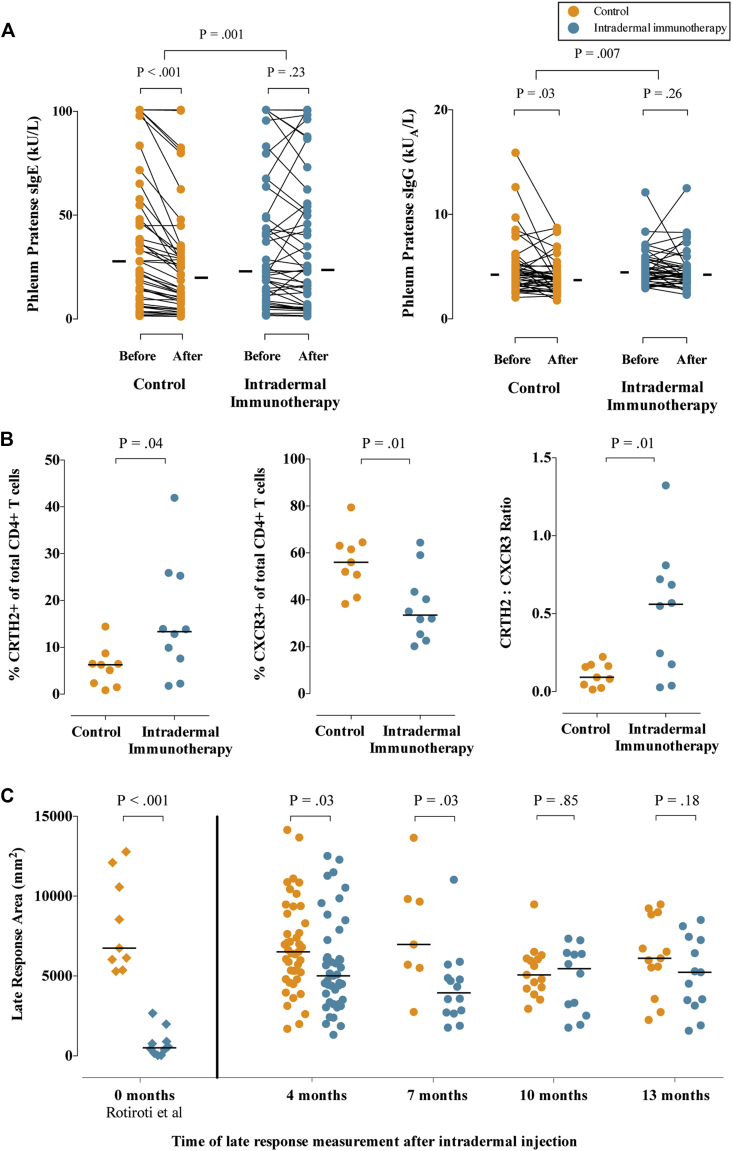
Fig 4.
Immunologic outcomes. A, Levels of P pratense–specific IgE and IgG before and after completion of 7 intradermal allergen or histamine control injections. B, Expression of CRTH2 (TH2 marker) and CXCR3 (TH1 marker) on CD4+ cells expanded from skin biopsy specimens (24 hours after skin challenge). C, Areas of cutaneous late-phase responses (24 hours after intradermal skin challenge) 4 months and either 7, 10, or 13 months after treatment (September 2013). Late-phase response suppression was shown in our previous study (Rotiroti et al5) immediately after 6 biweekly intradermal injections. Solid bars represent median values. P values for pretreatment and posttreatment serology comparisons are based on the Wilcoxon signed-rank test, and those for between-group IgE and IgG levels are based on analysis of covariance. P values in Fig 4, B and C, are based on the Mann-Whitney U test.