Abstract
Stroke is an important risk factor for bone fracture. We showed previously that bone fracture at the acute stage of ischemic stroke worsens, and activation of α-7 nicotinic acetylcholine receptor (α-7 nAchR) improves, stroke recovery by attenuating inflammation. We hypothesized that activation of α-7 nAchR also improves the blood-brain barrier (BBB) integrity. Permanent distal middle cerebral artery occlusion (pMCAO) was performed on C57BL/6J mice followed by tibia fracture 1 day later. Mice were treated with 0.8 mg/kg PHA 568487 (PHA, α-7 nAchR-specific agonist), 6 mg/kg methyllycaconitine (MLA, α-7 nAchR antagonist), or saline 1 and 2 days after pMCAO. Brain water content, the expression of monoamine oxidase B (MAO-B) and tight junction protein (claudin-5) were assessed. We found that tibia fracture increased water content in the ischemic stroke brain (p=0.006) and MAO-B positive astrocytes (p<0.001). PHA treatment reduced water content and MAO-B positive astrocytes, and increased claudin-5 expression in stroke and stroke+tibia fracture mice (p<0.05), while MLA had the opposite effect. Our findings suggest that in addition to inhibiting inflammation, activation of α-7 nAchR also reduces brain edema, possibly through diminished astrocyte oxidative stress and improved BBB integrity. Thus, the α-7 nAchR-specific agonist could be developed into a new therapy for improving recovery of patients with stroke or stroke+bone fracture.
Keywords: Ischemic stroke, Blood-brain barrier integrity, Claudin-5, Oxidative stress, PHA
Introduction
Stroke is one of the leading causes of death and disability worldwide and an important risk factor for bone fracture [1]. Stroke patients have a 2 to 4-fold higher risk of bone fracture than the general population. Post-stroke bone fracture increases mortality in older patients [2, 3]. Our previous studies showed that in mice, bone fracture after ischemic stroke exacerbates stroke-related brain injury and behavioral deficits [4], suggesting that post-stroke bone fracture has a negative impact on stroke outcomes. Understanding the underlying mechanisms of brain injury caused by post-stroke bone fracture can lead to a novel target for developing neuroprotective strategies.
Ischemic stroke causes neuronal injury through a complex pathological process involving multiple biological pathways [5]. A major and severe complication of ischemic stroke is brain edema, which exacerbates the brain injury and promotes clinical deterioration [6]. The main cause of brain edema is the loss of the blood-brain barrier (BBB) integrity that results in water accumulation in the brain [7]. The effect of post-stroke fracture on brain edema has not been analyzed.
Astrocytes are one of the important components of BBB. Alternation of monoamine metabolism in astrocytes can cause oxidative stress and alter function, leading to BBB dysfunction and brain edema [8]. Maintaining BBB integrity is crucial to prevent the influx of monoamines into the brain parenchyma [9]. Monoamine oxidases (MAO) are a family of enzymes that catalyze the oxidative deamination of monoamines and regulate the monoamine levels in the brain. A high level of monoamine can also increase MAO activity. There are two types of MAO in the brain (A and B); MAO-B activity is linked to oxidative stress in tissues [10].
Stroke triggers a cascade of inflammatory pathways. Excessive inflammation during the acute phase of ischemic stroke exacerbates brain damage; conversely, reducing the inflammation decreases brain damage and improves functional recovery [11]. We showed in our previous study that tibia fracture shortly before or after ischemic stroke enhances inflammation in the peri-infarct region [4, 12, 13]. Therefore, we chose to analyze whether inhibition of inflammatory pathway will reduce stroke injury in stroke-only and stroke+bone fracture subjects.
Nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channels, consisting of multiple, diverse subtypes. Alpha-7 (α-7) nAChR is one of the most widely-distributed subtypes throughout the central nervous system and on the surface of systemic macrophages [14–19]. Modulation of α-7 nAchR regulates inflammation [20, 21] and oxidative stress [22]. We have previously shown that α-7 nAchR agonist treatment attenuates neuroinflammation, oxidative stress, and brain injury in mice with stroke, or stroke and bone fracture [12, 23].
In the present study, we tested the hypothesis that post-stroke bone fracture aggravates BBB disruption, brain edema, and MAO-B expression in astrocytes, and that activation of α-7 nAchR reduces astrocyte MAO-B level and brain edema.
Materials and Methods
Animals
All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco, and conformed to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
C57BL/6J male mice (10–12 weeks old; Jackson Laboratory, Bar Harbor, ME) were randomly assigned to each treatment group. The experimenters were blinded to the treatments and data analysis.
Permanent Middle Cerebral Artery Occlusion (pMCAO) for Stroke Model
The left middle cerebral artery was permanently occluded through electrocoagulation using the method described in our previous study [4, 12]. Briefly, animals were anesthetized with 2% isoflurane inhalation. A 1.0 cm vertical skin incision was made between the left orbit and ear to expose the temporal bone. A 2.0 mm2 hole was drilled precisely over the region of the middle cerebral artery (MCA) followed by removal of dura mater. The MCA was then permanently occluded using electrical coagulation just proximal to the pyriform branch. Animals were placed on a thermal blanket (37 ± 0.5°C feedback-controlled by rectal temperature) for maintenance of body temperature throughout the surgical procedure. Successful occlusion of MCA was confirmed by laser Doppler flowmeter (Vasamedics Inc., Little Canada, MN). Animals were excluded from subsequent experiments if the surface cerebral blood flow in the ischemic core was >15% of the baseline, or if a massive bleeding caused by artery injuries occurred. Two intraperitoneal injections of buprenorphine (0.1 mg/kg of body weight) (0.3 mg in 100 µl saline) were given at the beginning of the surgery and 4 h after. Animals were allowed to recover spontaneously under warm conditions. Control mice were subjected to craniotomy without arterial occlusion but with the same amount and duration of anesthesia and the same amount of buprenorphine used for stroke mice.
Tibia Fracture Surgery for Bone Fracture Model
Twenty-four hours after the pMCAO procedure, animals were anesthetized with 2% isoflurane inhalation. Under aseptic surgical conditions, animals received an open tibia fracture of the right hind limb with an intramedullary fixation, as reported previously [12]. Animals were placed on a thermal blanket (37 ± 0.5°C feedback-controlled by rectal temperature) for maintenance of body temperature throughout the surgical procedure. Two intraperitoneal injections of buprenorphine (0.1 mg/kg of body weight) (0.3 mg in 100 µl saline) were given at the beginning of the surgery and 4 h after.
Experimental Groups and Design
Mice were randomly assigned to 10 groups listed in Table 1, 6 mice per group. Mice subjected to sham pMCAO and sham tibia fracture (wild-type), pMCAO and sham tibia fracture (stroke) or tibia fracture with sham pMCAO (tibia fracture) served as control. An α-7 nAchR selective agonist, PHA 568487 (PHA, Tocris Bioscience, Bristol, UK), or α-7 nAchR antagonist methyllycaconitine (MLA, Sigma, St Louis, MO, USA), was administered to the mice. PHA and MLA were diluted in 0.9% saline. Based on the findings in our previous study, we injected PHA (0.8 mg/kg) or MLA (6 mg/kg) on days 1 and 2 after pMCAO (Fig. 1), because we found in our previous study that injection of PHA and MLA after pMCAO with these doses and at these time points yielded the best effect on infarct volume and behavior tests [12]. Several studies have shown that PHA-568487 has rapid brain penetration [24–26]. In humans, PHA-568487 was excreted within the first 48 hours after a single dose of [3H]1 (40 mg, 163 µCi) [27, 28].
Table 1.
Experimental groups
| Group | Injury type | Treatment | N |
|---|---|---|---|
| 1 | sham pMCAO + sham tibia fracture (wild-type) | saline | 6 |
| 2 | sham pMCAO + tibia fracture (tibia fracture) | saline | 6 |
| 3 | sham pMCAO + tibia fracture (tibia fracture) | PHA | 6 |
| 4 | sham pMCAO + tibia fracture (tibia fracture) | MLA | 6 |
| 5 | pMCAO + sham tibia fracture (stroke) | saline | 6 |
| 6 | pMCAO + sham tibia fracture (stroke) | PHA | 6 |
| 7 | pMCAO + sham tibia fracture (stroke) | MLA | 6 |
| 8 | pMCAO + tibia fracture (stroke+tibia fracture) | saline | 6 |
| 9 | pMCAO + tibia fracture (stroke+tibia fracture) | PHA | 6 |
| 10 | pMCAO + tibia fracture (stroke+tibia fracture) | MLA | 6 |
Fig.1. Experimental design.
Tibia fracture was performed one day after pMCAO. Drugs were injected intra-peritoneally (i.p.) 1 (first injection) and 2 (second injection) days after pMCAO. Brain samples were collected 3 days after pMCAO. D: day.
Determination of Brain Edema
Brain edema was evaluated by measuring the brain water content, as previously described [29]. Briefly, the brain samples were collected three days after the pMCAO or sham pMCAO procedure. Both hemispheres were weighed before and after drying at 100 °C for 48 hours. The percentage of water content was calculated as 100 × (wet weight-dry weight)/wet weight.
Immunofluorescent Staining
Mice were anesthetized with isoflurane inhalation and 4% paraformaldehyde (PFA) perfusion. Brain samples were collected, frozen in dry ice, and cut into 20 µm thick sections (CM1900 Cryostat, Leica, Wetzlar, Germany). Three coronal sections (200µm apart) from each brain were immunostained with primary antibodies against glial fibrillary acidic protein (GFAP, astrocyte marker, 1:500, Invitrogen, Carlsbad, CA), MAO-B (1:100, Santa Cruz Biotechnology, Santa Cruz, CA), Claudin-5 (1:25, Invitrogen, Carlsbad, CA) and lectin (endothelial cell marker, 1:100, Vector Lab, Burlingame, CA). Following overnight incubation, the samples were incubated for 90 minutes with secondary antibodies: Alexa-594 or Alexa-488 IgG (Alexa 594 for red and Alexa 488 for green, 1:2000; Invitrogen, Carlsbad, CA). Vessels were stained by incubating sections overnight at 4 °C in 2g/ml with fluorescein lycopersicon esculentum lectin (Vector Laboratories Burlingame, CA). Negative controls were performed on sections collected from mice subjected to stroke and tibia fracture without applying any primary antibodies (refer to Supplementary Material Fig. 1). Images were taken using a fluorescent microscope (Nikon Microphoto-SA, Melville, NY) and analyzed using Image J software (National Institutes of Health, Bethesda, MD).
Statistical Analysis
Group-level summaries of brain water content, MAO-B positive astrocytes, and claudin-5 are expressed as mean±standard deviation (SD). We compared the brain water content of the four saline groups (Table 1) using one-way ANOVA. For each of the three outcome measures, we performed a two-way ANOVA with an interaction term; injury and treatment were the two independent variables tested. Groups 2–10 (Table 1) were included in the brain water content analysis, and groups 5–10, in the MAO-B positive astrocytes and claudin-5 analyses. For post-hoc pairwise comparisons of groups, we adjusted for multiple comparisons via Tukey’s method. We considered p-values less than 0.05 to be significant. Data analysis was conducted using Stata 13.1 (StataCorp. 2013. Stata Statistical Software: Release 13, College Station, TX: StataCorp LP).
Results
Tibia Fracture Increased Brain Edema in the Ischemic Stroke Brain
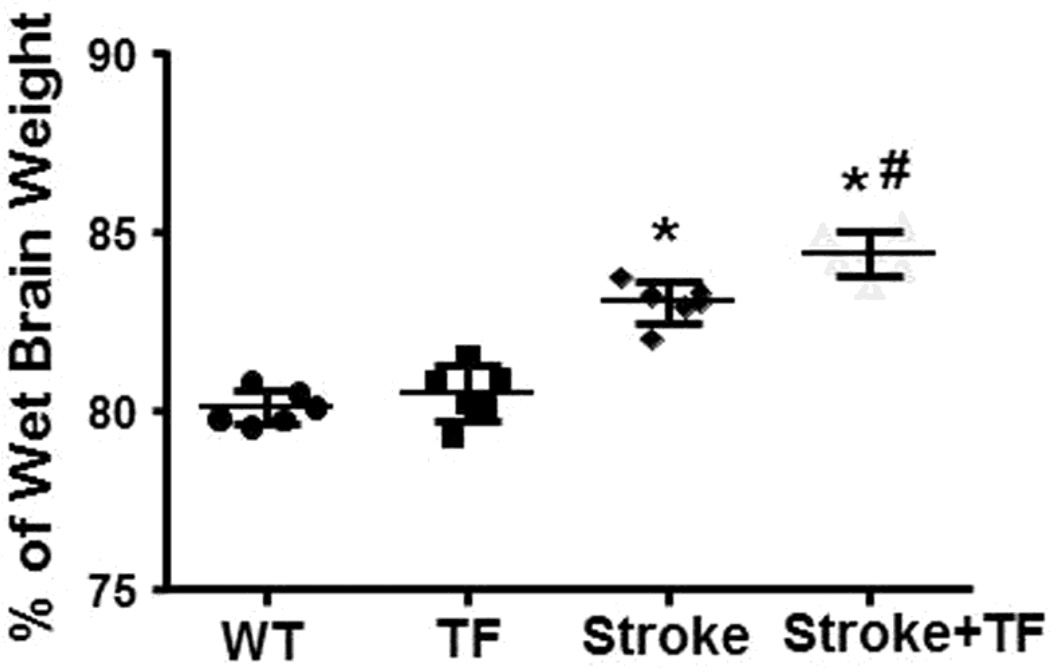
To analyze whether post-stroke tibia fracture enhances brain edema, we measured the brain water content. The water content in the uninjured contralateral hemisphere was similar among groups (P=0.24; refer to Supplementary Material Fig. 2). In the ipsilateral hemisphere of stroke brain, tibia fracture alone did not increase brain water content (80.1±0.5% of wet brain weight versus 80.5±0.8%, p=0.324, Fig. 2). The stroke group (83.0±0.6%) had more brain water content than the WT group (80.1±0.5%, p<0.001, Fig. 2). Post-stroke tibia fracture further increased water content in the stroke brain (84.4±0.6%, p=0.006), thereby indicating that tibia fracture after stroke does increase brain water content.
Fig. 2. Tibia fracture increased water content in the stroke brain.
WT: mice subjected to sham pMCAO and sham tibia fracture; TF: mice subjected to sham pMCAO and tibia fracture; Stroke: mice subjected to pMCAO and sham tibia fracture; Stroke+TF: mice subjected to both pMCAO and tibia fracture. *p<0.001 compared with WT group; #p=0.006 compared with stroke group.
PHA Treatment Reduced Brain Edema in Mice Subjected to Stroke Alone or Stroke+Tibia Fracture
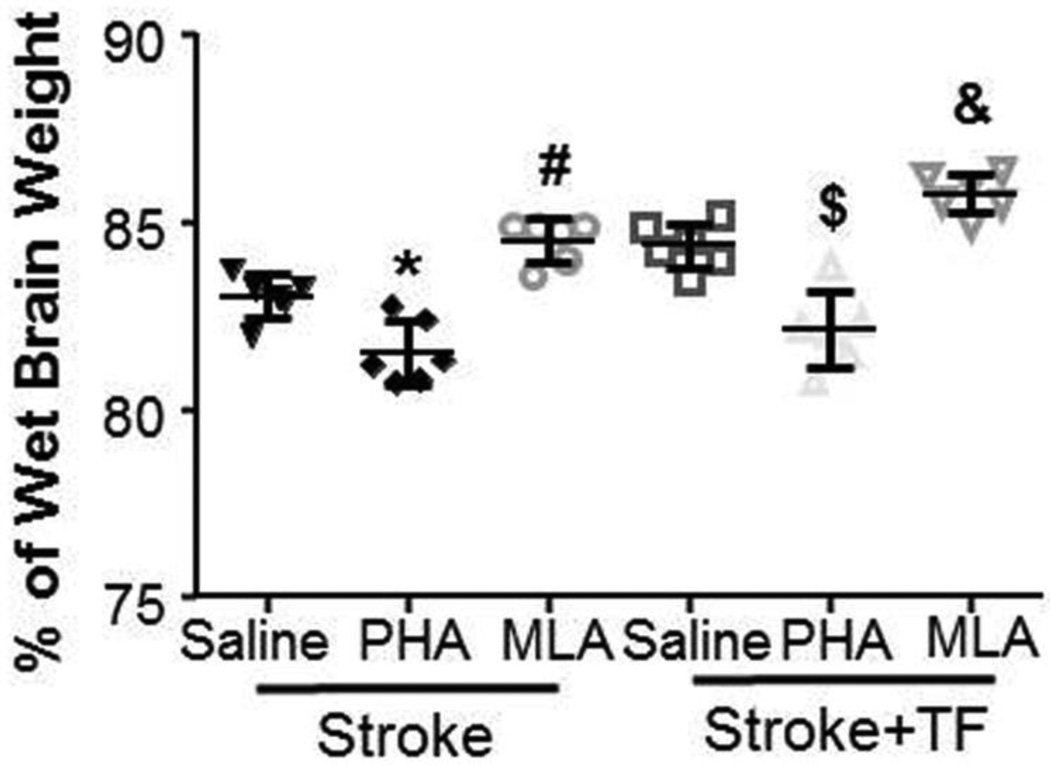
To assess if activation of α-7 nAchR reduces brain edema and inhibition of α-7 nAchR enhances brain edema of mice subjected to stroke or stroke+tibia fracture, mice were treated with PHA or MLA 1 and 2 days after stroke. Brain water content was measured 3 days post-stroke (Fig. 1). The two-way ANOVA analysis showed a significant interaction between injury type and treatment (p<0.001). The treatments had no noticeable effect on the brain water content of the tibia fracture group (p=1.00; refer to Supplementary Material Fig. 3) but strongly affected the stroke and stroke+tibia fracture groups (Fig. 3). Compared with the saline-treated group (83.0±0.6%), PHA reduced (81.5±0.8%, p=0.023) and MLA increased water content in the ipsilateral hemisphere (84.5%±0.6%, p=0.021) in stroke-only mice. A similar pattern was also observed in the stroke+tibia fracture groups. Compared with the saline-treated group (84.4±0.6%), the PHA group had significantly lower (82.2±1.0%, p<0.001) water content, while that of the MLA-treated group was significantly higher (85.8±0.5%, p=0.042) (Fig 3). Therefore, activation of α-7 nAchR reduced brain edema in stroke and stroke+tibia fracture mice.
Fig. 3. PHA treatment reduced brain edema.
Stroke: mice subjected to pMCAO and sham tibia fracture; Stroke+TF: mice subjected to both pMCAO and tibia fracture. *: p=0.023 and #: p=0.021 vs. saline-treated stroke mice. $: p<0.001 and &: p=0.042 vs. saline-treated stroke+tibia group.
Tibia Fracture Increased and PHA Treatment Decreased MAO-B Positive Astrocytes
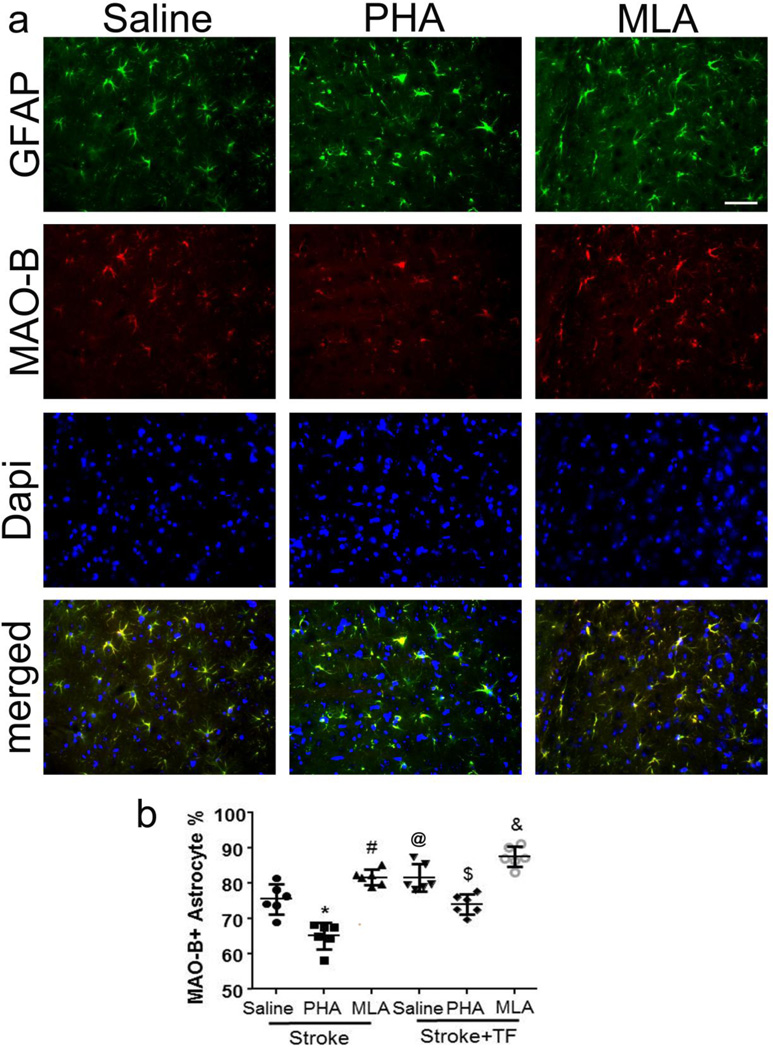
We next determined whether bone fracture increased astrocyte oxidative stress by quantifying MAO-B positive astrocytes in the peri-infarct region. The overall ANOVA F-test for this outcome was significant (p<0.001). Compared with stroke-only mice (75.4±4.3%), stroke+tibia fracture mice had more MAO-B positive astrocytes (81.7±3.9%, p=0.045, Fig. 4). PHA treatment reduced MAO-B positive astrocytes in the peri-infarct regions in both mice subjected to stroke-only (PHA vs. saline: 65.1±3.7% vs. 75.5±4.3%, p<0.001, Fig. 4) and mice subjected to stroke+tibia fracture (PHA vs. saline: 74.0±2.9% vs. 81.6±3.9%, p=0.007, Fig. 4). However, stroke+tibia fracture mice still had more MAO-B positive astrocytes than stroke-only mice after PHA treatment (p=0.001), suggesting that the oxidative stress in stroke+tibia fracture mice was more severe. MLA treatment increased the number of MAO-B positive astrocytes in the peri-infarct region of stroke (MLA vs. saline: 81.7±2.2% vs. 75.5±4.3%, p=0.038) and stroke+tibia fracture (MLA vs. saline: 87.6±2.9% vs. 81.6±3.9%, p=0.049) mice (Fig. 4).
Fig. 4. Fewer MAO-B+ astrocytes in the peri-infarct region of PHA-treated mice.
(a) Representative images. GFAP (green): glial fibriallary acidic protein (astrocyte marker). Scale bar: 50 µm. Nuclei were counterstained blue using Dapi. (b) Quantification of MAO-B+ astrocytes. TF: tibia fracture. @: p=0.045 compared to saline-treated stroke mice; *: p<0.001 compared with saline-treated stroke mice; #p<0.038 compared with saline-treated stroke mice; $:p=0.007 compared with saline-treated stroke+tibia fracture mice; &: p=0.049 compared with saline-treated stroke+tibia fracture mice.
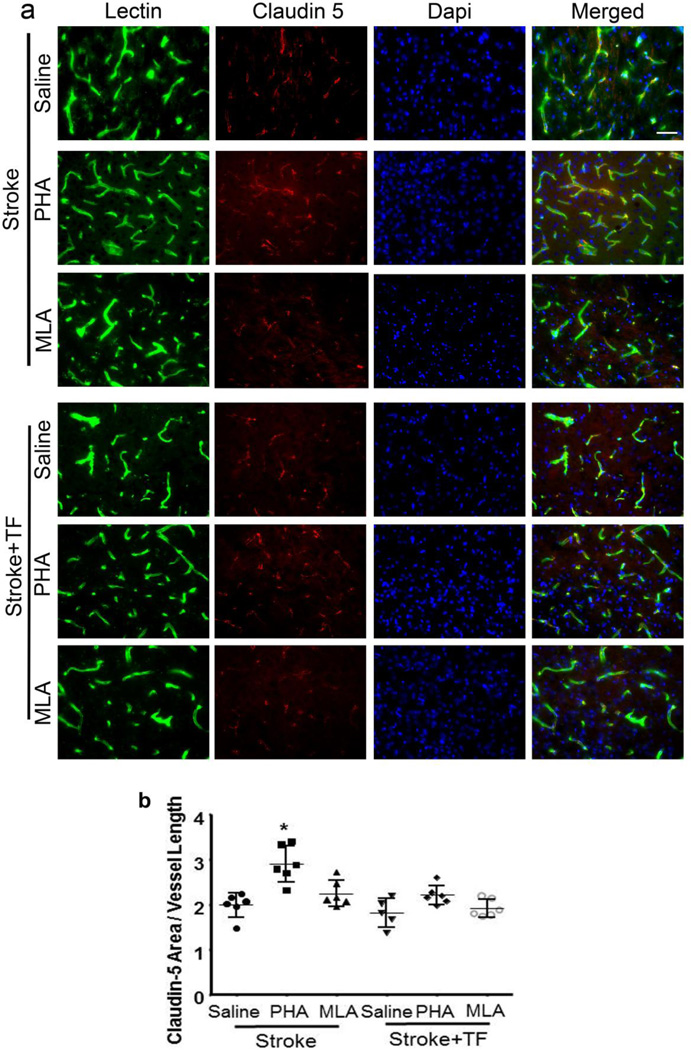
PHA Treatment Increased Tight Junction Protein Expression
To investigate the effect of PHA on tight junction protein expression, we analyzed claudin-5 expression in the peri-infarct region. The overall ANOVA F-test for this outcome was significant (p<0.001). We found that in stroke-only mice, PHA treatment increased claudin-5 expression (PHA vs. saline: 1122±156 mm2/mm of vessel length vs. 767±104, p<0.001, Fig. 5). MLA treatment did not change claudin-5 expression (p=0.62 compared with saline-treated). Neither PHA nor MLA altered claudin-5 expression significantly in the peri-infact region of mice subjected to ischemic stroke+tibia fracture (Fig. 5b), which could have been the result of more severe inflammation and BBB breakdown in mice with stroke+tibia fracture.
Fig. 5. PHA treatment increased Claudin-5 expression in the stroke-only brain.
(a) Representative images. Vessels were visualized by lectin staining (green). Claudin-5 positive staining is shown in red. Nuclei were counterstained blue using Dapi. Scale bar: 50 µm. (b) Bar graph shows quantification. TF: tibia fracture. *: p<0.001 compared with saline-treated stroke mice.
Discussion
In this study, we found that tibia fracture exacerbates brain edema and increases MAO-B expression in astrocytes in the ischemic stroke brain. Activation of α-7 nAchR through PHA treatment significantly ameliorated brain edema, which is associated with reduction of MAO-B expression in the peri-infarct region. PHA treatment increased the expression of Claudin-5 in the peri-infarct region of mice subjected to stroke only.
Clinical reports indicate that many stroke victims (approximately 70,000 in the United States) suffer from bone fracture within the first year after their stroke [30] and have a poorer outcome than those without bone fracture [4]. Our previous studies in mice demonstrate that tibia fracture after ischemic stroke exacerbates stroke-related brain injury and behavioral deficits [4]. We showed in this study that tibia fracture after ischemic stroke increases brain edema, which has been observed to occur as early as three hours after MCA occlusion in a rat MCAO model, reaching the maximum level on the third day and then gradually diminishing thereafter [31]. Bone fracture has also been shown to exacerbate brain edema and worsen the outcomes of traumatic brain injury in a multitrauma mouse model [32, 33]. Brain edema plays a critical role in neuronal damage, and clinical deterioration is often associated with brain ischemia. Therefore, reduction of brain edema could improve the outcomes of patients with stroke or stroke+tibia fracture.
Oxidative stress is an important element in the brain injury at the onset and progression of ischemic stroke [34, 35]. We showed previously that tibia fracture after ischemic stroke exacerbates oxidative stress by increasing the level of NADPH oxidase and NF-κb activity and reducing the expression of anti-oxidant genes [12]. In this study, we found that tibia fracture increases MAO-B expression in astrocytes in the peri-infarct region. Many studies have shown that MAO-derived H2O2 contributes to the oxidative stress and ischemic brain injury. Reduction of MAO-B expression has a neuroprotective effect. MAO-B inhibitors prevent the production of reactive oxygen species and brain injury after ischemia/reperfusion [36, 37].
Astrocytes are the main components of the blood-brain barrier (BBB) and participate in regulating BBB integrity and blood flow [38]. Therefore, oxidative stress in astrocytes can cause BBB breakdown and brain edema. We showed previously that activation of α-7 nAchR attenuates neuroinflammation; oxidative stress and brain injury in mice with stroke and bone fracture [12]. The therapeutic benefits of α-7 nAchR in ischemia-induced brain injury has also been observed in other animal models [21, 23, 39] and several other neurological disorders [40], such as Alzheimer’s disease and Parkinson’s disease [41, 42]. It has been shown in experimental intracerebral hemorrhage models that the α-7 nAChR agonists attenuates peri-hematomal edema and improves functional outcome [19, 43]. In this study, we demonstrated that α-7 nAchR specific agonist PHA reduced brain edema and MAO-B positive astrocytes in the peri-infarct regions of both stroke and stroke+tibia fracture mice, suggesting that reduction of astrocyte-oxidative stress could be one of the underlying mechanisms that contribute to PHA reduction of brain edema.
Disruption of the BBB is considered a major cause of edema. Tight junction proteins are the main components of BBB, and play a vital role in restricting BBB permeability. Claudin-5 is one of the important tight-junctional proteins contributing to the "sealing" of the tight junctions [44]. The expression of claudin-5 significantly decreased in a pMCAO rodent model [45, 46], which was responsible for increased BBB permeability and secondary brain edema. Recent studies have shown that tibia fracture exacerbates traumatic brain BBB disruption and edema [32, 33]. However, our study showed that tibia fracture post-stroke did not reduce claudin-5 expression further when compared with stroke-only mice. In addition, PHA treatment increased claudin-5 expression in stroke-only mice, but not in mice with stroke+tibia fracture. These results suggest that other mechanisms, such as astrocyte oxidative stress, are involved in the enhanced brain edema of mice with stroke+tibia fracture.
This study was conducted only on mice with tibia fracture one day after stroke. Because bone fracture can happen any time after stroke, the findings in this study may not apply to patients who experience bone fracture months after the stroke. However, based on the data published by Kanis et al, the estimated incidence of having a bone fracture within 24 hours of a stroke is 2.4–3.6/100,000, which is about 1–1.5% of stroke patients [30]. Thus, about 7,000–11,000 patients in the United States and 167,000–250,000 worldwide will experience a fall-fracture within the first day of the stroke. A study conducted in the U.S. confirmed that, compared to a nonstroke population, the hazard ratio of suffering from a hip fracture within the first 24 hours after stroke diagnosis is significantly higher (3.9, 95% CI of 2.1–7.3) [47]. A retrospective review of more than 400,000 surgical patients revealed that stroke is an independent risk factor for poor outcome after orthopedic bone surgery, but not abdominal aortic surgery [48]. Therefore, understanding the underlying mechanisms of brain injury caused by bone fracture at the acute stage of stroke can help in developing innovative neuroprotective strategies that may benefit a substantial number of patients. In a future study, we will determine the influence of bone fracture occurring months after the stroke on recovery.
In summary, along with our previous findings [12], the results of this study show that in ischemic stroke-only and ischemic stroke+tibia fracture mice, activation of α-7 nAchR reduces ischemic injury associated with: (1) heightened expression of the anti-oxidant gene; (2) reduced microglia/macrophage infiltration and M1/M2 microglia/macrophage ratio; (3) decreased pro-oxidative NADPH oxidase [12], MAO expression in astrocytes, and NF-κb activity; (4) increased tight-junction protein; and (5) diminished brain edema. Thus, activation of α-7 nAchR could be a therapeutic option for improving the outcomes of patients with stroke and bone fracture. Future studies are still needed to determine whether our findings can be successfully applied to human situations.
Supplementary Material
Acknowledgments
We thank members of the UCSF BAVM Study Project (http://avm.ucsf.edu) for their support, and Voltaire Gungab for assistance with manuscript preparation.
Funding
This study was supported by grants to H. S. from the National Institutes of Health (R01 NS027713, R01 HL122774 and R21 NS083788), and from the Michael Ryan Zodda Foundation and the UCSF Research Evaluation and Allocation Committee (REAC).
References
- 1.Sennerby U, Melhus H, Gedeborg R, Byberg L, Garmo H, Ahlbom A, Pedersen NL, Michaelsson K. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302(15):1666–1673. doi: 10.1001/jama.2009.1463. [DOI] [PubMed] [Google Scholar]
- 2.Frost SA, Nguyen ND, Black DA, Eisman JA, Nguyen TV. Risk factors for in-hospital post-hip fracture mortality. Bone. 2011;49(3):553–558. doi: 10.1016/j.bone.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25(5):457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 4.Degos V, Maze M, Vacas S, Hirsch J, Guo Y, Shen F, Jun K, van Rooijen N, Gressens P, Young WL, Su H. Bone fracture exacerbates murine ischemic cerebral injury. Anesthesiology. 2013;118(6):1362–1372. doi: 10.1097/ALN.0b013e31828c23f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toni D, Fiorelli M, Gentile M, Bastianello S, Sacchetti ML, Argentino C, Pozzilli C, Fieschi C. Progressing neurological deficit secondary to acute ischemic stroke. A study on predictability, pathogenesis, and prognosis. Arch Neurol. 1995;52(7):670–675. doi: 10.1001/archneur.1995.00540310040014. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42(11):3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oishi R, Itoh Y, Nishibori M, Watanabe T, Nishi H, Saeki K. Effect of MCI-186 on ischemia-induced changes in monoamine metabolism in rat brain. Stroke. 1989;20(11):1557–1564. doi: 10.1161/01.str.20.11.1557. [DOI] [PubMed] [Google Scholar]
- 9.Lasbennes F, Lacombe P, Seylaz J. Effect of monoamine oxidase inhibition on the regional cerebral blood flow response to circulating noradrenaline. Brain Res. 1988;454(1–2):205–211. doi: 10.1016/0006-8993(88)90819-0. [DOI] [PubMed] [Google Scholar]
- 10.Palencia G, Garcia E, Osorio-Rico L, Trejo-Solis C, Escamilla-Ramirez A, Sotelo J. Neuroprotective effect of thalidomide on MPTP-induced toxicity. Neurotoxicology. 2015:4782–4787. doi: 10.1016/j.neuro.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Hayakawa K, Qiu J, Lo EH. Biphasic actions of HMGB1 signaling in inflammation and recovery after stroke. Ann N Y Acad Sci. 2010:120750–120757. doi: 10.1111/j.1749-6632.2010.05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han Z, Li L, Wang L, Degos V, Maze M, Su H. Alpha-7 nicotinic acetylcholine receptor agonist treatment reduces neuroinflammation, oxidative stress and brain injury in mice with ischemic stroke and bone fracture. J Neurochem. 2014;131(4):498–508. doi: 10.1111/jnc.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Kang S, Zou D, Zhan L, Li Z, Zhu W, Su H. Bone fracture pre-ischemic stroke exacerbates ischemic cerebral injury in mice. PLoS One. 2016;11(4):e0153835. doi: 10.1371/journal.pone.0153835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zoli M, Pistillo F, Gotti C. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology. 2015;96(Pt B):302–311. doi: 10.1016/j.neuropharm.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Su X, Lee JW, Matthay ZA, Mednick G, Uchida T, Fang X, Gupta N, Matthay MA. Activation of the alpha7 nAChR reduces acid-induced acute lung injury in mice and rats. Am J Respir Cell Mol Biol. 2007;37(2):186–192. doi: 10.1165/rcmb.2006-0240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duris K, Manaenko A, Suzuki H, Rolland WB, Krafft PR, Zhang JH. alpha7 nicotinic acetylcholine receptor agonist PNU-282987 attenuates early brain injury in a perforation model of subarachnoid hemorrhage in rats. Stroke. 2011;42(12):3530–3536. doi: 10.1161/STROKEAHA.111.619965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lightfoot AP, Kew JN, Skidmore J. Alpha7 nicotinic acetylcholine receptor agonists and positive allosteric modulators. Prog Med Chem. 2008:46131–46171. doi: 10.1016/S0079-6468(07)00003-3. [DOI] [PubMed] [Google Scholar]
- 18.Takada-Takatori Y, Kume T, Sugimoto M, Katsuki H, Sugimoto H, Akaike A. Acetylcholinesterase inhibitors used in treatment of Alzheimer's disease prevent glutamate neurotoxicity via nicotinic acetylcholine receptors and phosphatidylinositol 3-kinase cascade. Neuropharmacology. 2006;51(3):474–486. doi: 10.1016/j.neuropharm.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Krafft PR, Altay O, Rolland WB, Duris K, Lekic T, Tang J, Zhang JH. alpha7 nicotinic acetylcholine receptor agonism confers neuroprotection through GSK-3beta inhibition in a mouse model of intracerebral hemorrhage. Stroke. 2012;43(3):844–850. doi: 10.1161/STROKEAHA.111.639989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamano R, Takahashi HK, Iwagaki H, Yoshino T, Nishibori M, Tanaka N. Stimulation of alpha7 nicotinic acetylcholine receptor inhibits CD14 and the toll-like receptor 4 expression in human monocytes. Shock. 2006;26(4):358–364. doi: 10.1097/01.shk.0000228168.86845.60. [DOI] [PubMed] [Google Scholar]
- 21.Neumann S, Shields NJ, Balle T, Chebib M, Clarkson AN. Innate immunity and inflammation post-stroke: an alpha7-nicotinic agonist perspective. Int J Mol Sci. 2015;16(12):29029–29046. doi: 10.3390/ijms161226141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarro E, Buendia I, Parada E, Leon R, Jansen-Duerr P, Pircher H, Egea J, Lopez MG. Alpha7 nicotinic receptor activation protects against oxidative stress via heme-oxygenase I induction. Biochem Pharmacol. 2015;97(4):473–481. doi: 10.1016/j.bcp.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Han Z, Shen F, He Y, Degos V, Camus M, Maze M, Young WL, Su H. Activation of alpha-7 nicotinic acetylcholine receptor reduces ischemic stroke injury through reduction of pro-inflammatory macrophages and oxidative stress. PLoS One. 2014;9(8):e105711. doi: 10.1371/journal.pone.0105711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beinat C, Banister SD, Herrera M, Law V, Kassiou M. The therapeutic potential of alpha7 nicotinic acetylcholine receptor (alpha7 nAChR) agonists for the treatment of the cognitive deficits associated with schizophrenia. CNS Drugs. 2015;29(7):529–542. doi: 10.1007/s40263-015-0260-0. [DOI] [PubMed] [Google Scholar]
- 25.Wishka DG, Walker DP, Yates KM, Reitz SC, Jia S, Myers JK, Olson KL, Jacobsen EJ, Wolfe ML, Groppi VE, Hanchar AJ, Thornburgh BA, Cortes-Burgos LA, Wong EH, Staton BA, Raub TJ, Higdon NR, Wall TM, Hurst RS, Walters RR, Hoffmann WE, Hajos M, Franklin S, Carey G, Gold LH, Cook KK, Sands SB, Zhao SX, Soglia JR, Kalgutkar AS, Arneric SP, Rogers BN. Discovery of N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide, an agonist of the alpha7 nicotinic acetylcholine receptor, for the potential treatment of cognitive deficits in schizophrenia: synthesis and structure--activity relationship. J Med Chem. 2006;49(14):4425–4436. [Google Scholar]
- 26.Walker DP, Wishka DG, Piotrowski DW, Jia S, Reitz SC, Yates KM, Myers JK, Vetman TN, Margolis BJ, Jacobsen EJ, Acker BA, Groppi VE, Wolfe ML, Thornburgh BA, Tinholt PM, Cortes-Burgos LA, Walters RR, Hester MR, Seest EP, Dolak LA, Han F, Olson BA, Fitzgerald L, Staton BA, Raub TJ, Hajos M, Hoffmann WE, Li KS, Higdon NR, Wall TM, Hurst RS, Wong EH, Rogers BN. Design, synthesis, structure-activity relationship, and in vivo activity of azabicyclic aryl amides as alpha7 nicotinic acetylcholine receptor agonists. Bioorg Med Chem. 2006;14(24):8219–8248. doi: 10.1016/j.bmc.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Shilliday FB, Walker DP, Gu C, Fang X, Thornburgh B, Fate GD, Daniels JS. Multiple species metabolism of PHA-568487, a selective alpha 7 nicotinic acetylcholine receptor agonist. Drug Metab Lett. 2010;4(3):162–172. [PubMed] [Google Scholar]
- 28.Shaffer CL, Gunduz M, Scialis RJ, Fang AF. Metabolism and disposition of a selective alpha(7) nicotinic acetylcholine receptor agonist in humans. Drug Metab Dispos. 2007;35(7):1188–1195. doi: 10.1124/dmd.106.014449. [DOI] [PubMed] [Google Scholar]
- 29.Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34(8):2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. [DOI] [PubMed] [Google Scholar]
- 30.Kanis J, Oden A, Johnell O. Acute and long-term increase in fracture risk after hospitalization for stroke. Stroke. 2001;32(3):702–706. doi: 10.1161/01.str.32.3.702. [DOI] [PubMed] [Google Scholar]
- 31.Gotoh O, Asano T, Koide T, Takamura K. Ischemic brain edema following occlusion of the middle cerebral artery in the rat. I: The time courses of the brain water, sodium and potassium contents and blood-brain barrier permeability to 125-I albumin. Stroke. 1985;16(1):101–109. doi: 10.1161/01.str.16.1.101. [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Guo Y, Wen D, Chen Y, Zhang G, Fan Z. Bone fracture enhances trauma brain injury. Scand J Immunol. 2016;83(1):26–32. doi: 10.1111/sji.12393. [DOI] [PubMed] [Google Scholar]
- 33.Shultz SR, Sun M, Wright DK, Brady RD, Liu S, Beynon S, Schmidt SF, Kaye AH, Hamilton JA, O'Brien TJ, Grills BL, McDonald SJ. Tibial fracture exacerbates traumatic brain injury outcomes and neuroinflammation in a novel mouse model of multitrauma. J Cereb Blood Flow Metab. 2015;35(8):1339–1347. doi: 10.1038/jcbfm.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu Y, Liu Q, Anrather J, Shi FD. Immune interventions in stroke. Nat Rev Neurol. 2015;11(9):524–535. doi: 10.1038/nrneurol.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chamorro A, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016 doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 36.Szilagyi G, Simon L, Wappler E, Magyar K, Nagy Z. (-)Deprenyl-N-oxide, a (-)deprenyl metabolite, is cytoprotective after hypoxic injury in PC12 cells, or after transient brain ischemia in gerbils. J Neurol Sci. 2009;283(1–2):182–186. doi: 10.1016/j.jns.2009.02.368. [DOI] [PubMed] [Google Scholar]
- 37.Eliash S, Speiser Z, Cohen S. Rasagiline and its (S) enantiomer increase survival and prevent stroke in salt-loaded stroke-prone spontaneously hypertensive rats. J Neural Transm (Vienna) 2001;108(8–9):909–923. doi: 10.1007/s007020170012. [DOI] [PubMed] [Google Scholar]
- 38.Pan Q, He C, Liu H, Liao X, Dai B, Chen Y, Yang Y, Zhao B, Bihl J, Ma X. Microvascular endothelial cells-derived microvesicles imply in ischemic stroke by modulating astrocyte and blood brain barrier function and cerebral blood flow. Mol Brain Res. 2016;9(1):63. doi: 10.1186/s13041-016-0243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guan YZ, Jin XD, Guan LX, Yan HC, Wang P, Gong Z, Li SJ, Cao X, Xing YL, Gao TM. Nicotine inhibits microglial proliferation and is neuroprotective in global ischemia rats. Mol Neurobiol. 2015;51(3):1480–1488. doi: 10.1007/s12035-014-8825-3. [DOI] [PubMed] [Google Scholar]
- 40.Conejero-Goldberg C, Davies P, Ulloa L. Alpha7 nicotinic acetylcholine receptor: a link between inflammation and neurodegeneration. Neurosci Biobehav Rev. 2008;32(4):693–706. doi: 10.1016/j.neubiorev.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leiser SC, Bowlby MR, Comery TA, Dunlop J. A cog in cognition: how the alpha 7 nicotinic acetylcholine receptor is geared towards improving cognitive deficits. Pharmacol Ther. 2009;122(3):302–311. doi: 10.1016/j.pharmthera.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Safieh-Garabedian B, Oz M, Bey RM, Shamaa F, Ashoor A, El-Agnaf OM, Saade NE. Involvement of the alpha7-nicotinic acetylcholine receptors in the anti-inflammatory action of the thymulin-related peptide (PAT) Neuroscience. 2013:250455–250466. doi: 10.1016/j.neuroscience.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 43.Sussman ES, Kellner CP, McDowell MM, Bruce SS, Heuts SG, Zhuang Z, Bruce RA, Claassen J, Connolly ES., Jr Alpha-7 nicotinic acetylcholine receptor agonists in intracerebral hemorrhage: an evaluation of the current evidence for a novel therapeutic agent. Neurosurg Focus. 2013;34(5):E10. doi: 10.3171/2013.2.FOCUS1315. [DOI] [PubMed] [Google Scholar]
- 44.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32(2):200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J, Fu B, Zhang X, Chen L, Zhang L, Zhao X, Bai X, Zhu C, Cui L, Wang L. Neuroprotective effect of bicyclol in rat ischemic stroke: down-regulates TLR4, TLR9, TRAF6, NF-kappaB, MMP-9 and up-regulates claudin-5 expression. Brain Res. 2013:152880–152888. doi: 10.1016/j.brainres.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y, Fu B, Zhang X, Zhao T, Chen L, Zhang J, Wang X. Paeonol pretreatment attenuates cerebral ischemic injury via upregulating expression of pAkt, Nrf2, HO-1 and ameliorating BBB permeability in mice. Brain Res Bull. 2014:10961–10967. doi: 10.1016/j.brainresbull.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Lakshminarayan K, Schissel C, Anderson DC, Vazquez G, Jacobs DR, Jr, Ezzeddine M, Luepker RV, Virnig BA. Five-year rehospitalization outcomes in a cohort of patients with acute ischemic stroke: medicare linkage study. Stroke. 2011;42(6):1556–1562. doi: 10.1161/STROKEAHA.110.605600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanders RD, Bottle A, Jameson SS, Mozid A, Aylin P, Edger L, Ma D, Reed M, Walters M, Lees KR, Maze M. Independent preoperative predictors of outcomes in orthopedic and vascular surgery: the influence of time interval between an acute coronary syndrome or stroke and the operation. Ann Surg. 2012;255(4):901–907. doi: 10.1097/SLA.0b013e31824c438d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.