Figure 1.
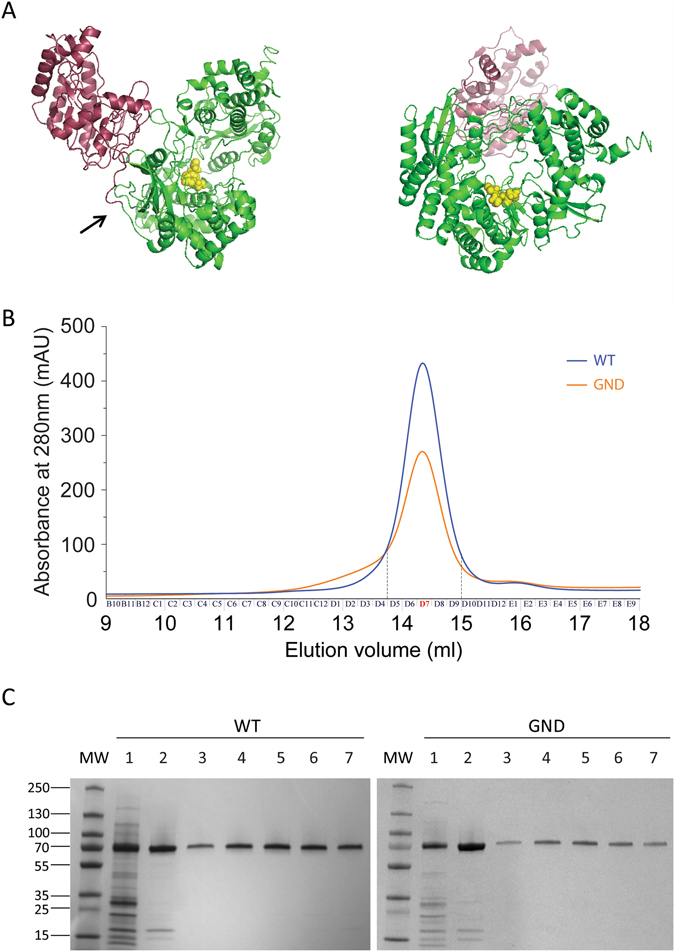
Structure prediction of NS5 and purification of ZIKV polymerase. (A) Structure prediction of the NS5 protein of ZIKV. The putative polymerase domain is in green and the MTase domain in purple. The arrow shows the limit between the MTase and the RdRp domain that was cloned. The three amino acids of the potential active site GDD appear as yellow spheres. Images were obtained following a 45° rotation. (B) Size exclusion chromatography profiles. Chromatograms correspond to the retention profile of the WT enzyme (blue) and catalytic mutant GND (orange). (C) SDS-PAGE analysis of polypeptides throughout the purification of WT (left) and catalytic mutant GND (right) ZIKV RdRp. MW corresponds to the molecular weight. Lane 1 corresponds to 4 µg of total protein pooled from the nickel column elution. Lane 2 corresponds to 3 µg of total protein pooled from the heparin column. Lanes 3 to 7 correspond to 0.4 µg of protein from fractions D5 to D9 obtained during size exclusion Proteins were stained with Coomassie Brillant blue R250.
