Figure 2.
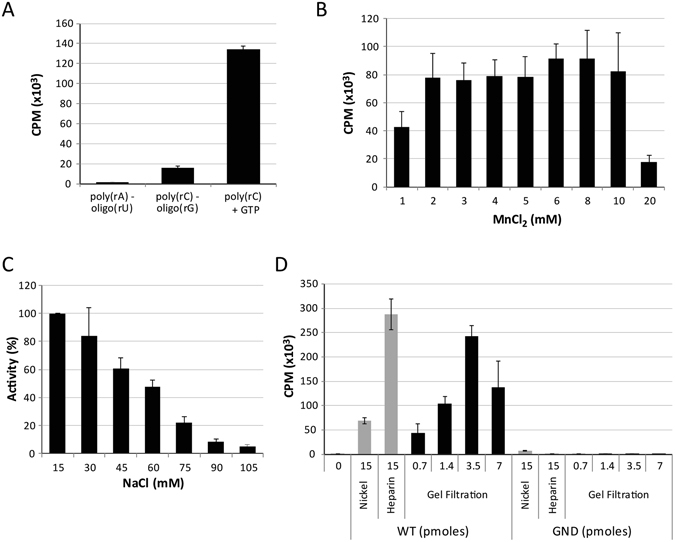
ZIKV RdRp enzymatic activities using homopolymeric templates. (A) Template utilization by ZIKV RdRp. Radionucleotide incorporation was monitored using 3.5 pmoles of WT enzyme (fraction D7 from gel filtration, see Fig. 1B) and various template-primer combinations. (B) Effect of the metal cofactor on nucleotide incorporation. (C) Impact of increasing concentration of NaCl on radionucleotide incorporation. The assay was conducted in conditions similar to panel A using the poly(rC) substrate and increasing concentrations of MnCl2 and NaCl, respectively. Because the RdRp was purified in a buffer containing NaCl, the lowest salt concentration reachable is 15 mM (no salt added). (D) De novo RNA synthesis activity of ZIKV RdRp throughout the purification stages. Pooled fractions eluted from the nickel column and from the heparin column were tested at 15 pmoles of total proteins for the WT and GND mutant polymerases. In parallel, fraction D7, corresponding to the main retention pic after gel filtration was assayed at 0.7, 1.4, 3.5 and 7 pmoles. Means and standard deviations derives from three independent determinations.
