Abstract
During initiation of conjugative transfer of DNA containing the transfer origin (oriT) of the promiscuous plasmid RP4, the proteins TraI, TraJ, and TraH interact and assemble a specialized nucleoprotein complex (the relaxosome) at oriT. The structure can be visualized on electron micrographs. Site- and strand-specific nicking at the transfer origin in vitro is dependent on the proteins TraI and TraJ and on Mg2+ ions. Substrate specificity is directed exclusively towards the cognate transfer origin: the RP4-specified TraJ protein cannot recognize the closely related oriT of plasmid R751. After nicking, TraI protein remains attached to the 5'-terminal 2'-deoxycytidyl residue at the nic site [Pansegrau, W., Ziegelin, G. & Lanka, E. (1990) J. Biol. Chem. 265, 10637-10644]. Nicking and relaxosome formation require supercoiled DNA. Thus, a complicated structure involving multiple plasmid-specified proteins and a defined region of DNA must be formed at the transfer origin to prepare the plasmid for generating the single strand to be transferred.
Full text
PDF

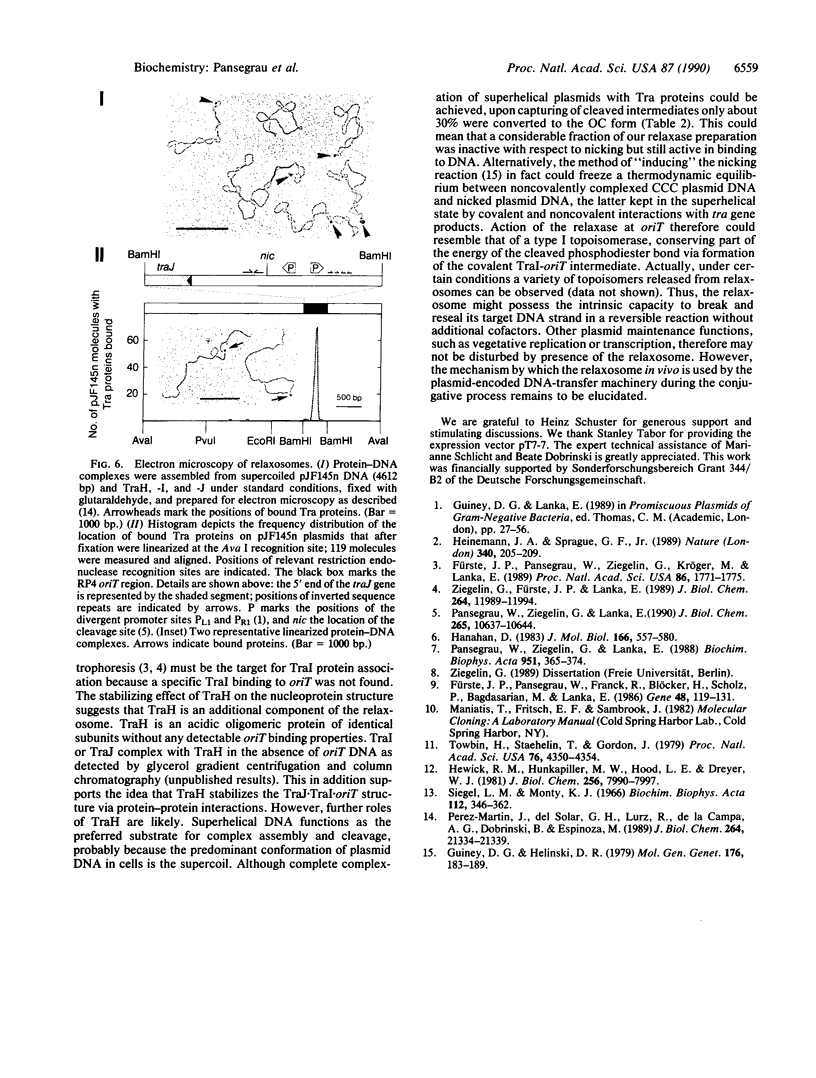
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Ziegelin G., Kröger M., Lanka E. Conjugative transfer of promiscuous IncP plasmids: interaction of plasmid-encoded products with the transfer origin. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1771–1775. doi: 10.1073/pnas.86.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney D. G., Helinski D. R. The DNA-protein relaxation complex of the plasmid RK2: location of the site-specific nick in the region of the proposed origin of transfer. Mol Gen Genet. 1979 Oct 3;176(2):183–189. doi: 10.1007/BF00273212. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Heinemann J. A., Sprague G. F., Jr Bacterial conjugative plasmids mobilize DNA transfer between bacteria and yeast. Nature. 1989 Jul 20;340(6230):205–209. doi: 10.1038/340205a0. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Pansegrau W., Ziegelin G., Lanka E. Covalent association of the traI gene product of plasmid RP4 with the 5'-terminal nucleotide at the relaxation nick site. J Biol Chem. 1990 Jun 25;265(18):10637–10644. [PubMed] [Google Scholar]
- Pansegrau W., Ziegelin G., Lanka E. The origin of conjugative IncP plasmid transfer: interaction with plasmid-encoded products and the nucleotide sequence at the relaxation site. Biochim Biophys Acta. 1988 Dec 20;951(2-3):365–374. doi: 10.1016/0167-4781(88)90108-x. [DOI] [PubMed] [Google Scholar]
- Pérez-Martín J., del Solar G. H., Lurz R., de la Campa A. G., Dobrinski B., Espinosa M. Induced bending of plasmid pLS1 DNA by the plasmid-encoded protein RepA. J Biol Chem. 1989 Dec 15;264(35):21334–21339. [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegelin G., Fürste J. P., Lanka E. TraJ protein of plasmid RP4 binds to a 19-base pair invert sequence repetition within the transfer origin. J Biol Chem. 1989 Jul 15;264(20):11989–11994. [PubMed] [Google Scholar]