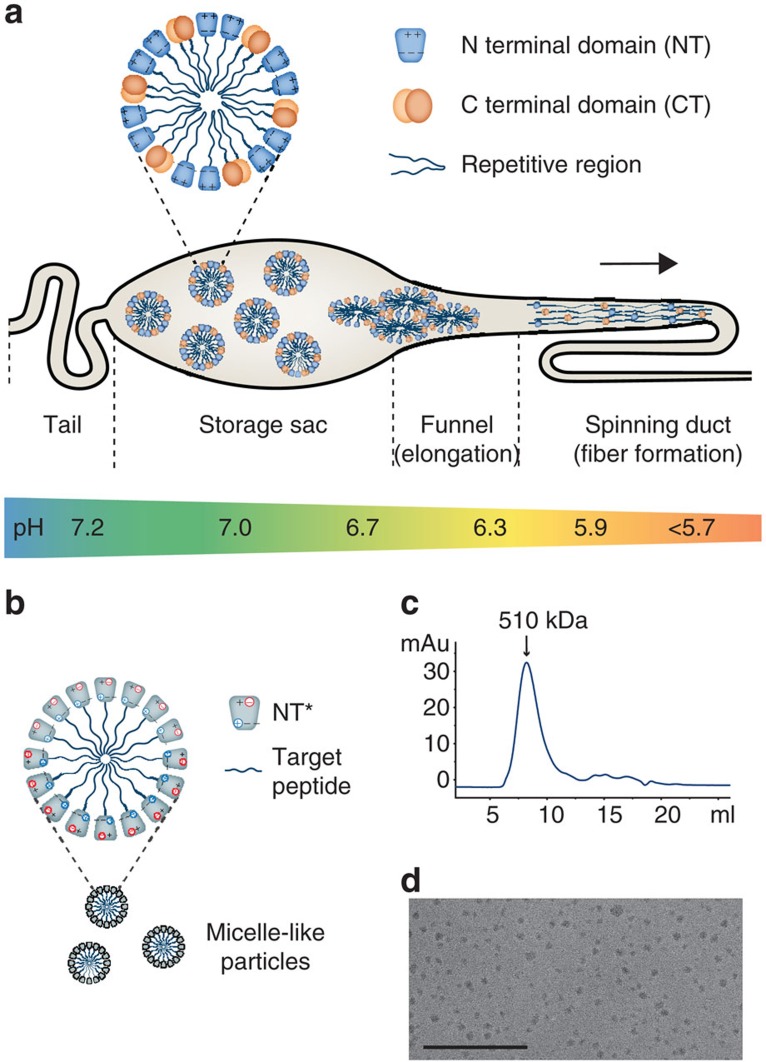
Figure 1. Spider silk proteins are stored as micellar structures—rationale of the current approach.
(a) Spidroins are synthesized in the tail region of the gland and stored at physiological pH in the sac. Premature aggregation may be prevented by formation of micelles, where the hydrophilic NT and CT domains sequester the hydrophobic and repetitive regions. The dipolar charge distribution of NT is illustrated by +and − signs. During passage through the spinning duct, the pH is gradually lowered, resulting in interconnection of spidroins through antiparallel dimerization of the NT domains. Assembly of spidroins gradually takes place due to changes in environmental factors and shear forces along the narrowing duct. At the exit point, the repetitive regions have arranged into strong fibres consisting of mainly β-sheets, making the spidroins inherently aggregation prone. (b) Recombinant production of hydrophobic and/or aggregation-prone peptides or proteins can be achieved using NT as a fusion tag that mediates solubility and shields hydrophobic/aggregation-prone regions from the aqueous surrounding within micelle-like particles. The mutant NT* is unable to dimerize at low pH due to a reduced dipolar charge distribution and is therefore able to mediate solubility in a wider pH range than NTwt (c) Size-exclusion chromatography of NT*-rSP-C33Leu shows that that the purified amphipathic fusion protein arrange into 510 kDa assemblies and (d) micelle-like particles around 10–15 nm in size are observed by TEM. Scale bar, 200 nm.