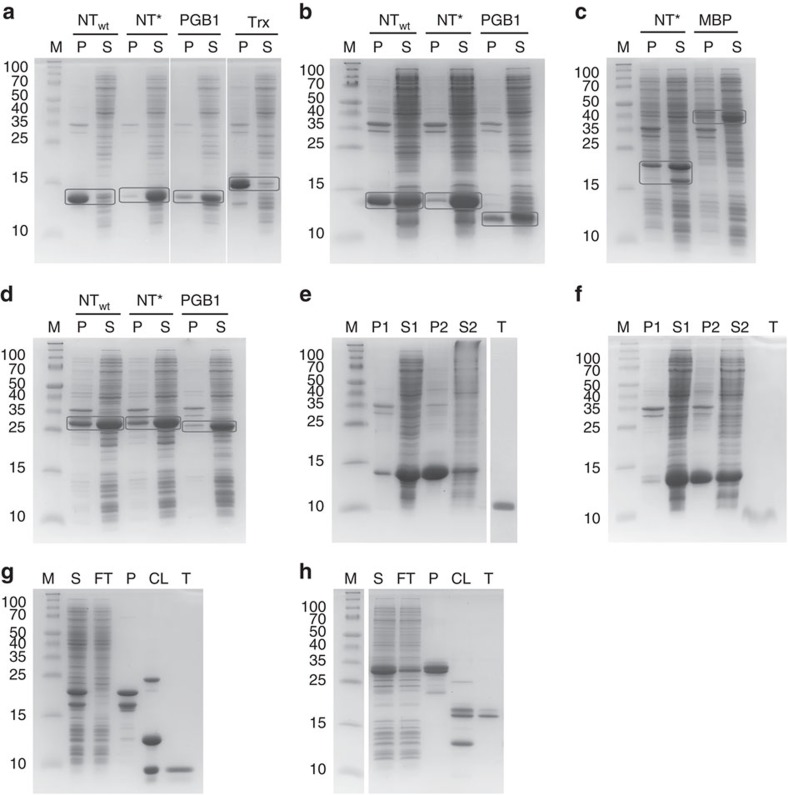
Figure 4. Solubility analysis of fusion proteins and subsequent purification of target peptides and protein.
Samples were analysed by SDS-PAGE and the molecular weights were compared to a protein standard (lane M). The molecular weights in kDa are given to the left of each gel figure. (a–d) Collected cells expressing peptides or protein in fusion with NTwt, NT*, PGB1, Trx or MBP were sonicated and centrifuged to separate the soluble (S) and insoluble (P) fractions. Representative gels are shown for (a) rSP-C33Leu, (b) rKL4, (c) rCCK-58 and (d) rfhSP-D fusion proteins. For each fusion protein, the bands corresponding to the soluble and insoluble fractions are boxed. (e–h) NT* fusion proteins were further used for purification using different strategies. Surfactant peptides were purified by a simple NaCl precipitation/ethanol extraction protocol (Fig. 5) as shown for (e) rSP-C33Leu and (f) rKL4. The lanes represent insoluble fraction (P1), soluble fraction (S1), pellet after first NaCl precipitation (P2), supernatant after first NaCl precipitation (S2) and purified target peptide (T). Standard Ni-sepharose chromatography was used for purification of (g) rCCK-58 and (h) rfhSP-D. The lanes represent supernatant after sonication (S), column flow-through (FT), purified fusion protein (P), cleavage products with 3C protease (CL) and purified target protein (T).