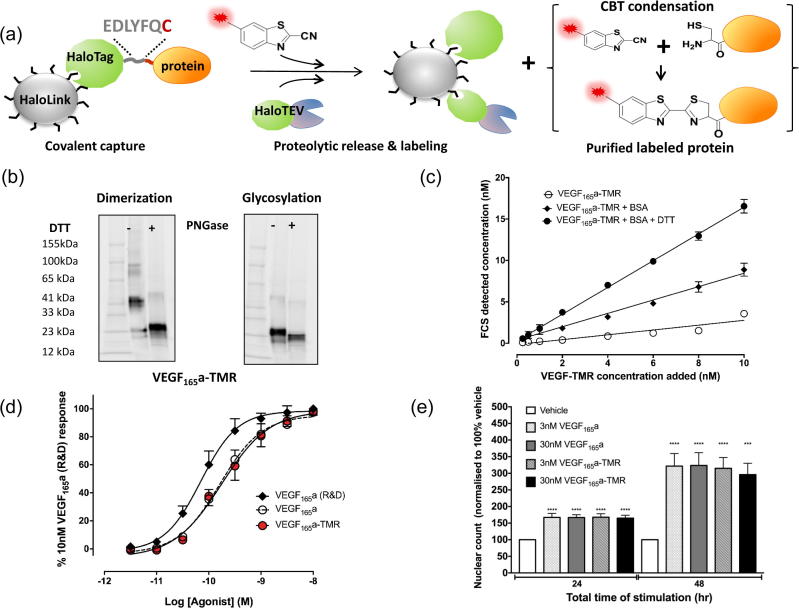
Fig. 1.
Synthesis and characterisation of purified VEGF165a-TMR. (a) Synthetic strategy for purification and labeling of VEGF165a-TMR. (b) Fluorescent SDS-PAGE analysis (of VEGF165a-TMR (Eex = 532 nm; Eem = 580 nm) in the presence or absence of 100 mM DTT and with or without deglycosylation by PNGase. (c) Influence of bovine serum albumin (0.1% BSA) and 10 mM DTT on VEGF165a-TMR concentrations measured using fluorescence correlation spectroscopy (FCS). Data are from 3 independent experiments and expressed as mean ± SEM. (d) Stimulation of NFAT luciferase production by HEK293T cells stably expressing VEGFR2 by VEGF165a (R&D Systems; closed circles), VEGF165a prepared identically to the fluorescent analogue (open circles) or fluorescent VEGF165a-TMR (red circles). Values represent mean ± SEM from 4 independent experiments from which quadruplicate determinations were made. Data are expressed as a percentage of the response to 10 nM VEGF165a (R&D Systems) obtained in each separate experiment. (e) Effect of VEGF165a and VEGFR165a-TMR on proliferation of human umbilical endothelial cells (HUVECs). Following stimulation with VEGF165a or VEGF165a-TMR (3 or 30 nM) for 24 or 48 h, HUVECs were fixed using 3% PFA/PBS and the nuclei stained using H33342 (2 mg/ml). Cells were imaged using a IX Micro widefield platereader at 4× magnification and nuclei were counted using a granularity algorithm (MetaXpress, Molecular Devices). Data are presented as fold increases in proliferation compared to vehicle treatment (mean ± S.E.M) and are pooled from 5 individual experiments. One way ANOVA was used to determine the statistical significance of ligand treatment when compared to vehicle only for both 24 and 48 h treatments (***P < 0.0002; ****P < 0.0001). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)