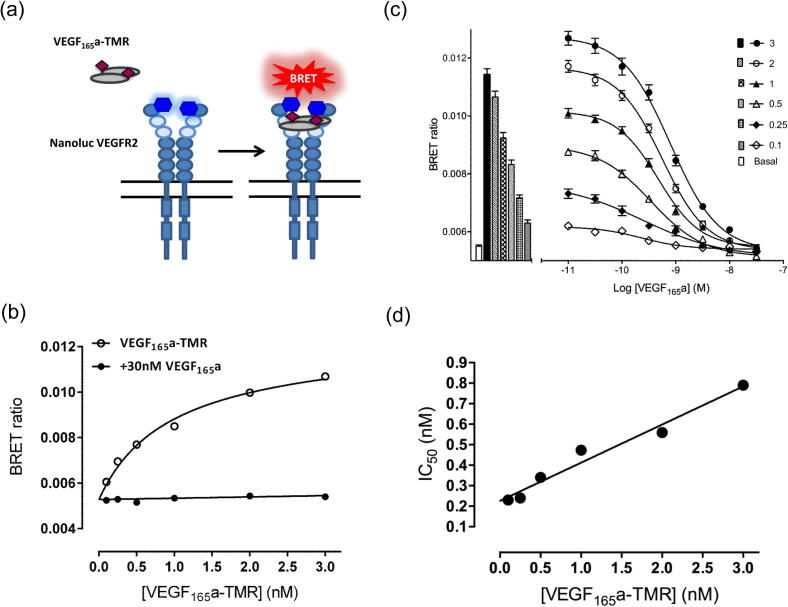
Fig. 3.
Binding characteristics of VEGF165a-TMR to VEGFR2 in HEK293T cells. (a) Schematic of the use of NanoBRET to investigate the binding of VEGF165a-TMR to an N terminal NanoLuc tagged VEGFR2 (NLuc-VEGFR2). (b) Saturation binding of increasing concentrations of VEGF165a-TMR in the presence or absence of unlabeled VEGF165a (30 nM; to define non-specific binding). Data are expressed as raw BRET ratios. VEGF165a-TMR (0.1–3 nM) and unlabeled VEGF165a (30 nM) were added simultaneously to triplicate wells and incubated for 60 min at 37 °C. Values represent mean ± S.E.M of five independent experiments performed independently of displacement experiments shown in (c). Where not shown, the error bars are within the size of the symbol. (c) Inhibition of the binding of VEGF165a-TMR (0.1–3 nM) to NanoLuc-tagged VEGFR2 by increasing concentrations of unlabeled VEGF165a. VEGF165a-TMR (0.1–3 nM) and unlabeled VEGF165a were added simultaneously and incubated for 60 min at 37 °C. Values represent mean ± S.E.M of five independent experiments. Bars represent the binding of VEGF165a-TMR obtained at each fluorescent ligand concentration in the absence of competing unlabeled VEGF165a. The open bar shows the BRET ratio obtained in the absence of either fluorescent or unlabeled ligand. (d) Linear regression analysis (R2 = 0.97; p < 0.001) of the relationship between IC50 value determined in (c) and the concentration of VEGF165a-TMR. The y intercept provides an estimate for the Ki of competing VEGF165a (0.22 nM) and the slope (0.19) represents the ratio Ki/KD yielding a value of 0.85 nM for the KD of VEGF165a-TMR.