Figure 2.
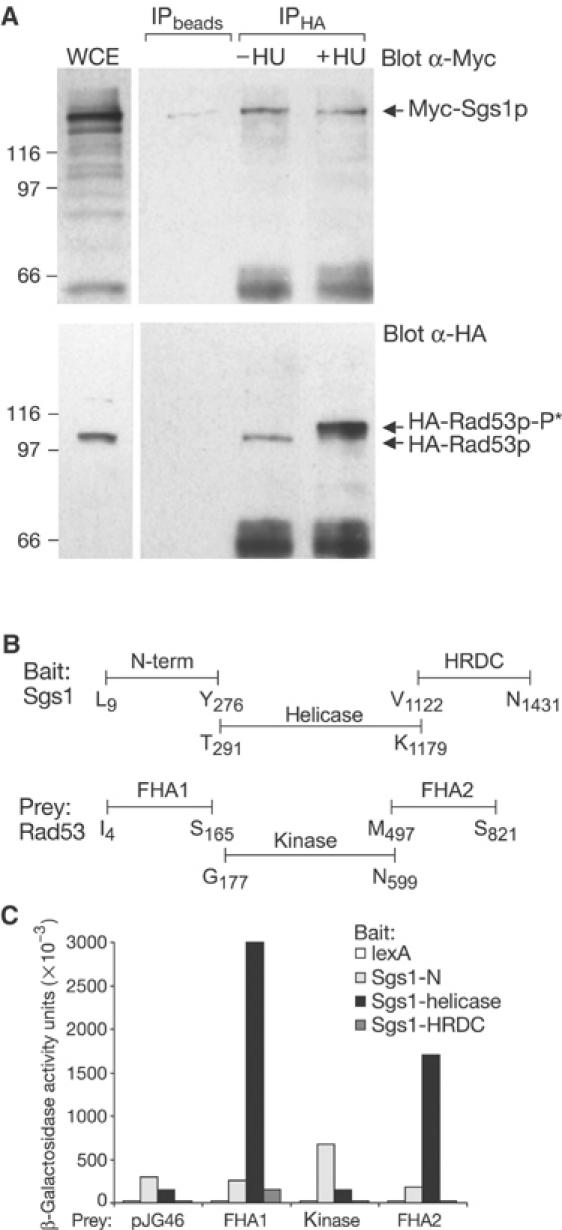
Specific interaction between Sgs1p and the Rad53p FHA1 domain. (A) Immunoprecipitation experiment performed with whole-cell extracts (WCEs) from GA-1142 cells (expressing Myc-tagged Sgs1p and HA-tagged Rad53p). WCEs were obtained from either random or HU-blocked cultures and α-HA (12CA5)-coupled Dynabeads were used for co-immunoprecipitation. Blots were probed with α-Myc (9E10) for Sgs1p (upper panel) or α-HA (Rad53, lower panel). (B) Scheme of Sgs1p and Rad53p domains used to fuse to the B42 activator domain in pJG46 or to the lexA DNA binding domain in pGAL-lexA. (C) The two-hybrid assay shows strong interactions between Sgs1-helicase and Rad53-FHA1 (20-fold), and Rad53-FHA2 (12-fold). Point mutations in FHA1 (R70A) or FHA2 (R605A) eliminate this interaction in pull-down assays (data not shown). β-Galactosidase units are described in Materials and methods.
