Figure 7.
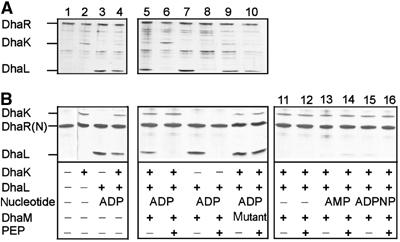
Association of DhaK and DhaL with full-length DhaR (A) and DhaR(N) signalling domain (B) is modulated by the phosphorylation state of DhaL∷ADP. Protein mixtures were preincubated, and complexes containing hexahistidine-tagged DhaR or DhaR(N) were affinity captured with Ni2+-NTA-beads, analyzed by denaturing polyacrylamide gel electrophoresis and visualized by silver staining. Lane 1, pull-down of purified DhaR with background; lanes 2 and 3, binary complexes of DhaK and DhaL∷ADP with DhaR; lane 4, competition between DhaK and DhaL∷ADP for DhaR; lanes 5–10, the PTS-dependent phosphorylation of DhaL∷ADP reduces DhaL affinity for DhaR. Phosphorylation of DhaL∷ADP requires PEP and active DhaM (lanes 6 and 8). The mutant DhaM(H9A, H169A, H430A) is not active (lanes 9 and 10). Lanes 11–16, DhaL apoenzyme (without ADP) and apoenzyme complemented with AMP or ADPPNP do not bind to DhaR (shown only with DhaR(N). The concentrations were as follows: DhaR (full length) 100 nM, DhaR(N) 100 nM, DhaK 300 nM, DhaL∷ADP 400 nM, apo-DhaL 400 nM, ADP 1 μM, AMP 1 μM, ADPNP 10 μM, DhaM 5 nM, PEP 1 mM, and EI and HPr 5 nM.
